Abstract
Background
We sought to analyze the impact of socioeconomic status (SES) on in‐hospital outcomes, cost of hospitalization, and resource use after acute ischemic stroke.
Methods and Results
We used the 2003–2011 Nationwide Inpatient Sample database for this analysis. All admissions with a principal diagnosis of acute ischemic stroke were identified by using International Classification of Diseases, Ninth Revision codes. SES was assessed by using median household income of the residential ZIP code for each patient. Quartile 1 and quartile 4 reflect the lowest‐income and highest‐income SES quartile, respectively. During a 9‐year period, 775 905 discharges with acute ischemic stroke were analyzed. There was a progressive increase in the incidence of reperfusion on the first admission day across the SES quartiles (P‐trend<0.001). In addition, we observed a significant reduction in discharge to nursing facility, across the SES quartiles (P‐trend<0.001). Although we did not observe a significant difference in in‐hospital mortality across the SES quartiles in the overall cohort (P‐trend=0.22), there was a significant trend toward reduced in‐hospital mortality across the SES quartiles in younger patients (<75 years) (P‐trend<0.001). The mean length of stay in the lowest‐income quartile was 5.75 days, which was significantly higher compared with other SES quartiles. Furthermore, the mean adjusted cost of hospitalization among quartiles 2, 3, and 4, compared with quartile 1, was significantly higher by $621, $1238, and $2577, respectively. Compared with the lowest‐income quartile, there was a significantly higher use of echocardiography, invasive angiography, and operative procedures, including carotid endarterectomy, in the highest‐income quartile.
Conclusions
Patients from lower‐income quartiles had decreased reperfusion on the first admission day, compared with patients from higher‐income quartiles. The cost of hospitalization of patients from higher‐income quartiles was significantly higher than that of patients from lowest‐income quartiles, despite longer hospital stays in the latter group. This might be partially attributable to a lower use of key procedures among patients from lowest‐income quartile.
Keywords: acute ischemic stroke, cost of illness, mortality, socioeconomic status, ZIP code
Introduction
Stroke is a leading cause of morbidity and mortality worldwide. Stroke was ranked as the seventh leading cause of disability adjusted life years lost in 2002 and has been projected to become the sixth leading cause by 2030.1–2 With the current progression in the incidence of strokes, the total number of strokes has been projected to increase to 18 million in 2015 and 23 million in 2030, in the absence of effective community‐based interventions.3 Socioeconomic disparities are widely prevalent in the treatment and the outcomes after cardiovascular and cerebrovascular disease including, acute ischemic stroke.4–9 Some of these disparities might be mediated by differences in biologic risk factors like blood pressure, diabetes, and lipid levels among patients from different socioeconomic strata.7 Disparities could also be related to delay in recognition of stroke symptoms, delay in presentation to the hospital, or differences in stroke etiology.8,10–12 In addition, few studies have demonstrated that significant socioeconomic disparities might exist in the use of treatments like thrombolysis and mechanical thrombectomy for acute ischemic stroke.5,8
There is emerging literature to suggest that the place of residence might play an important role in the outcome after acute ischemic stroke.7,13–18 It has been suggested that the neighborhood effects might not be completely mediated or moderated by individual socioeconomic, behavioral, or biologic risk factors.7 In this context, a number of cultural, clinical, economic, geographic, and access‐related issues could influence the time from symptom onset to first medical contact. While these variables are difficult to individually quantify, socioeconomic status (SES) measured by ZIP code could serve as a useful surrogate. Several prior studies have validated this approach for imputing individual SES in an epidemiologic setting.19–23 Residential ZIP code–based classification of SES may reflect the aggregate characteristics of its residents and the prevailing healthy and unhealthy habits, which serves to provide an insight into environmental attributes (like availability and accessibility of healthcare resources) that may have a direct or indirect impact on its residents’ health. In this analysis, we aimed to evaluate the characteristics and outcomes in patients presenting with acute ischemic stroke according to the SES. In addition, we evaluated the changing trends in administration of timely reperfusion therapy along with in‐hospital costs and resource use for patients presenting with acute ischemic stroke during the past decade.
Methods
Data Source
Data were obtained from the Nationwide Inpatient Sample (NIS) database from 2003 to 2011. The NIS is sponsored by the Agency for Healthcare Research and Quality (AHRQ) as a part of Healthcare Cost and Utilization Projection (HCUP). The NIS contains discharge level data from ≈8 million hospitalizations annually from ~1000 hospitals across the United States. This database is designed to represent a 20% stratified sample of all hospitals in the country. Criteria used for stratified sampling of hospitals into the NIS include location (urban or rural), teaching status, geographic region, patient volume, and hospital ownership.
Study Population
The NIS database provides up to 15 diagnoses and 15 procedures for each hospitalization record for 2003–2009. The number of diagnoses coded in the database was expanded to 25 for 2010–2011. All these have been coded by using the standard International Classification of Diseases, Ninth Edition, Clinical Modification (ICD‐9‐CM) codes. The first diagnosis in the database is referred to as the “principal diagnosis” and is considered the primary reason for admission to the hospital. All hospitalizations with the principal diagnosis of acute ischemic stroke were included in our study. These were identified by using ICD‐9 CM codes of 346.60 to 346.63, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434, 434.01, 434.1, 434.10, 434.11, 434.9, 434.90, 434.91, and 436. We used the HCUP Clinical Classification Software (CCS) to identify patient comorbidities and procedures.24 CCS has been developed by the AHRQ to cluster patient diagnoses and procedures into a manageable number of clinically meaningful categories.24 Patients undergoing thrombolysis were identified by using ICD‐9 CM codes of 99.10 or V45.88. Patients undergoing mechanical thrombectomy were identified by using the ICD‐9 code of 39.74. Baseline characteristics available for analysis included age, sex, race, primary source of payment, weekday versus weekend admission, and Elixhauser comorbidities defined by AHRQ along with other clinically relevant comorbidities (smoking and dyslipidemia).25–26 Hospital characteristics such as region (northeast, midwest, south, west), bed size (small, medium, large), location (rural, urban), and teaching status were also included.
The primary variable of interest was the SES assessed by using the median income of the patient's residential ZIP code. The NIS has classified each ZIP code into quartiles based on median household income of each ZIP code: quartile 1: $1 to $37 999, quartile 2: $38 000 to $47 999, quartile 3: $48 000 to $62 999, quartile 4: ≥$63 000. Residential ZIP code–based classification of SES is known to reflect aggregate characteristics of its residents and provide an insight into environmental attributes (eg, available healthcare resources) that may have a direct or indirect impact on its residents’ health. We chose to use the ZIP code–based classification to reflect on SES because of its successful validation in prior studies, along with the fact that it reflects aggregate characteristics over individual characteristics, which often govern healthcare delivery.19–23
Study Outcomes
In‐hospital mortality and reperfusion therapy administered on the first admission day were treated as co‐primary outcomes. In addition to these primary outcomes, we analyzed the differences in cost of hospitalization stratified by income‐based ZIP code quartile. The NIS database provides the total charges for hospital stay that were claimed by the respective hospital. The total charges of each hospital stay were converted to cost estimates by using the group average all‐payer in‐hospital cost and charge information from the detailed reports by hospitals to the Centers for Medicare & Medicaid Services. All costs and charges were converted to projected estimates for 2011, after accounting for annual inflation rates based on consumer price index data available from the Bureau of Labor Statistics.27 In addition, we analyzed the need for discharge to nursing facilities including long‐term acute care or subacute nursing facility extended care facility, stratified by the SES quartile, which was assumed to be a measure of resultant disability among the patients admitted with acute ischemic stroke. Further, to understand the reasons for cost differences between the various SES quartiles, we analyzed differences in several resource use variables such as length of stay (LOS), use of echocardiogram (procedure CCS: 193, ICD‐9 code: 37.28), invasive cerebral angiography (procedure CCS: 188), carotid endarterectomy (procedure CCS: 51), and any invasive operating room procedure performed on the head/neck region (procedure CCS: 59 and 51).
Sensitivity and Subgroup Analyses
We conducted a sensitivity analysis by comparing the study outcomes between the study groups after removing all patients who presented as a “transfer” from another healthcare facility. In addition, we compared the study outcomes between several patient strata including males versus females, whites versus nonwhites, and elderly (age ≥75 years) versus nonelderly (age <75 years) patients.
Statistical Analysis
Continuous variables are presented as mean±SD, and categorical variables are presented as proportions. To compare the means of continuous variables among ≥3 categories, we used 1‐way ANOVA. In cases of significant differences detected by using ANOVA, pairwise comparisons were performed by using Bonferroni correction for multiple comparisons. The χ2 test was used for comparison of categorical variables. To offset the statistically significant differences between baseline characteristics that may be created by large sample sizes, we have also reported standardized differences between the lowest‐income and the highest‐income quartiles. Standardized differences between means of continuous variables were calculated by using Cohen's d statistic, and those between proportions of categorical variables were calculated by using Cramer's V statistic.28
Survey statistics traditionally used to analyze complex semirandom survey designs were used to analyze these data. Because the data from NIS represent a collection of scattered hospital clusters, analysis was structured to account for a complex, multistage, probability sampling. NIS recommends the use of “strata” for constructing analysis clusters, which include geographic census region, hospital ownership, teaching status, urban/rural location, and bed size. Further, the analysis is further stratified into individual hospitals, which serve as primary sampling units for the analysis. In the NIS database, each hospital admission is linked to a “discharge weight” that can be used to calculate projected national estimates for all hospital‐related outcomes, after accounting for the hierarchical structure of the dataset.
Multivariable hierarchical logistic regression analysis was used to compare outcomes between the ZIP code quartiles. For this analysis, we used the variable “nis_straum” as the stratum, the variable “hospid” as the primary sampling unit (clustering variable), and the variable “discwt” as the sampling weight. The analysis of all outcomes has been presented after adjusting for age, sex, race, 29 Elixhauser comorbidities, and other relevant comorbidities, including smoking, dyslipidemia, primary payor, year of admission, and hospital characteristics. We assessed for the interaction between race and ZIP code–based SES in all regression models with the study outcomes as dependent variables. The lowest‐income SES quartile (quartile 1) has been used as the reference category for all comparisons.
All statistical analyses were performed by using the statistical software Stata v 13.1 (StataCorp). All statistical tests were 2‐tailed; a value of P<0.05 was considered significant. All data available from the HCUP have been deidentified, and hence the analysis is exempt from the federal regulations for the protection of human research participants. Therefore, an institutional review board approval was not necessary. The dataset was obtained from the AHRQ after completing the data use agreement with HCUP.
Results
During a 9‐year period (2003–2011), a total of 775 905 discharges with a principal diagnosis of acute ischemic stroke were analyzed. Based on the cluster design of the dataset, the estimates derived from the analysis represent outcomes from 3.8 million US patients admitted with acute ischemic stroke. Table 1 demonstrates the baseline characteristics of the entire study population, stratified by the SES quartiles. There were small yet statistically significant differences in the mean age and sex distribution across the SES quartiles. Overall, 56.9% of all patients residing in the lowest‐income SES quartile were white compared with 79.8% of patients in the highest‐income SES quartile (P<0.001). In addition, patients in the highest‐income SES quartile were more likely to have private insurance as the primary payment source compared with those in the lower‐income quartiles (P<0.001).
Table 1.
Baseline Characteristics Stratified by ZIP Code–Based Socioeconomic Status (SES) Quartile
| Characteristics | SES Quartile 1 | SES Quartile 2 | SES Quartile 3 | SES Quartile 4 | P Value | Standardized Difference (Quartile 4−Quartile 1)* |
|---|---|---|---|---|---|---|
| N | 230 028 | 206 523 | 182 991 | 156 363 | ||
| Median household income, US$ | 1 to 38 999 | 39 000 to 47 999 | 48 000 to 62 999 | ≥63 000 | ||
| Mean (SE) age, y | 69.8 (14.6) | 71.5 (14.5) | 72.1 (14.4) | 73.3 (14.4) | <0.001 | 0.23* |
| Females, n (%) | 125 438 (54.6) | 111 562 (54.0) | 98 182 (53.7) | 83 176 (53.2) | <0.001 | 0.01 |
| Race, n (%)* | ||||||
| White | 104 038 (56.9) | 117 740 (74.9) | 110 071 (76.5) | 104 357 (79.8) | <0.001 | 0.24* |
| Black | 52 559 (28.8) | 21 517 (13.7) | 15 470 (10.8) | 10 332 (7.9) | <0.001 | 0.26* |
| Other | 26 246 (14.4) | 17 929 (11.4) | 18 271 (12.7) | 16 141 (12.3) | <0.001 | 0.03 |
| Weekend admission | 58 096 (25.3) | 52 703 (25.5) | 46 820 (25.6) | 40 046 (25.6) | 0.03 | 0.004 |
| Primary expected payer, n (%) | ||||||
| Medicare | 151 924 (66.2) | 140 437 (68.1) | 123 852 (67.8) | 106 726 (68.3) | <0.001 | 0.02 |
| Medicaid | 21 392 (9.3) | 12 666 (6.1) | 8840 (4.8) | 5336 (3.4) | <0.001 | 0.11* |
| Private insurance | 36 388 (15.9) | 38 445 (18.7) | 39 007 (21.4) | 37 230 (23.8) | <0.001 | 0.10* |
| Uninsured | 12 944 (5.6) | 9177 (4.5) | 6640 (3.6) | 4033 (2.6) | <0.001 | 0.07 |
| Other | 6866 (3.0) | 5404 (2.6) | 4378 (2.4) | 2871 (1.8) | <0.001 | 0.04 |
| Hospital characteristics, n (%) | ||||||
| Region | ||||||
| Northeast | 29 096 (12.7) | 29 338 (14.2) | 33 027 (18.1) | 44 209 (28.3) | <0.001 | 0.19* |
| Midwest | 43 290 (18.8) | 56 699 (27.5) | 47 942 (26.2) | 30 350 (19.4) | <0.001 | 0.01 |
| South | 131 341 (57.1) | 86 737 (42.0) | 61 923 (33.8) | 41 957 (26.8) | <0.001 | 0.31* |
| West | 26 301 (11.4) | 33 749 (16.3) | 40 099 (21.9) | 39 847 (25.5) | <0.001 | 0.18* |
| Bed size | ||||||
| Small | 25 059 (11.0) | 26 850 (13.1) | 23 299 (12.8) | 18 855 (12.1) | <0.001 | 0.02 |
| Medium | 53 779 (23.6) | 48 755 (23.8) | 44 339 (24.3) | 42 527 (27.2) | <0.001 | 0.04 |
| Large | 149 389 (65.5) | 129 520 (63.1) | 114 538 (62.9) | 94 732 (60.7) | <0.001 | 0.05 |
| Urban location | 167 993 (73.6) | 166 642 (81.2) | 171 706 (94.3) | 153 936 (98.6) | <0.001 | 0.33* |
| Teaching hospital | 96 652 (42.4) | 76 326 (37.2) | 77 757 (42.7) | 72 996 (46.8) | <0.001 | 0.04 |
| Comorbidities, n (%) | ||||||
| Diabetes | 81 756 (35.9) | 66 644 (32.5) | 56 263 (31.0) | 43 551 (28.0) | <0.001 | 0.08 |
| Hypertension | 176 920 (77.6) | 155 594 (75.8) | 138 665 (76.3) | 117 946 (75.7) | <0.001 | 0.02 |
| Smoking | 47 600 (20.7) | 42 132 (20.4) | 37 049 (20.3) | 27 813 (17.8) | <0.001 | 0.04 |
| Drug abuse | 6212 (2.7) | 3239 (1.6) | 2333 (1.3) | 1383 (0.9) | <0.001 | 0.06 |
| Alcohol abuse | 9486 (4.2) | 6989 (3.4) | 5875 (3.2) | 4290 (2.8) | <0.001 | 0.02 |
| Obesity | 14 711 (6.5) | 12 686 (6.2) | 11 272 (6.2) | 8247 (5.3) | <0.001 | 0.02 |
| Dyslipidemia | 85 402 (37.1) | 81 544 (39.5) | 76 700 (41.9) | 66 696 (42.7) | <0.001 | 0.06 |
| Congestive heart failure | 32 962 (14.5) | 28 319 (13.8) | 24 633 (13.6) | 20 869 (13.4) | <0.001 | 0.02 |
| Peripheral vascular disease | 17 601 (7.7) | 16 511 (8.0) | 15 495 (8.5) | 13 244 (8.5) | <0.001 | 0.01 |
| Chronic pulmonary disease | 35 338 (15.5) | 30 955 (15.1) | 25 882 (14.2) | 19 686 (12.6) | <0.001 | 0.04 |
| Chronic renal failure | 23 895 (10.5) | 19 471 (9.5) | 17 432 (9.6) | 14 441 (9.3) | <0.001 | 0.02 |
| Mean (SD) No. of Elixhauser comorbidities | 2.51 (1.49) | 2.43 (1.47) | 2.46 (1.51) | 2.41 (1.51) | <0.001 | 0.07 |
All quartiles were based on median household income of the respective ZIP code. Data are expressed as number (percentage) except where specified. Quartile 1, $1 to $38 999; quartile 2, $39 000 to $47 999; quartile 3, $48 000 to $63 000; quartile 4, ≥$63 000. Quartile 1 reflects the lowest‐income quartile, and quartile 4 reflects the highest‐income quartile.
Standardized differences were calculated between quartile 1 and quartile 4 by using Cohen's d statistic for continuous variables and Cramer's V statistic for categorical variables.
A small standardized difference (Cohen's d: 0.2 to 0.5, Cramer's V: 0.1 to 0.3).
Data on race were available on 614 671 discharges only.
A moderate standardized difference (Cohen's d: 0.5 to 0.8, Cramer's V: 0.3 to 0.5).
The differences in the distribution of traditional atherosclerotic risk factors between the different SES quartiles are also illustrated in Table 1. There were small but statistically significant decreases in the prevalence of diabetes, hypertension, smoking, obesity, peripheral vascular disease, and chronic renal failure across the SES quartiles (P<0.001 for all comparisons). However, comparison of standardized differences between the lowest‐income and highest‐income quartiles demonstrated that all these statistically significant differences were small differences.
Figure 1 demonstrates the incidence and adjusted odds ratio (OR) for the study outcomes across the SES quartiles. Overall rates of reperfusion therapy were small. The use of thrombolysis or thrombectomy on first admission day across the SES quartiles was 2.2%, 2.6%, 3.0%, and 3.7%, respectively (P‐trend<0.001). On adjusted analysis, there was a trend indicating a progressively higher reperfusion therapy among patients from higher‐income SES quartiles, compared with those from lower‐income quartiles. Despite this, there was no significant difference in in‐hospital mortality rates across the SES quartiles. The incidence of in‐hospital mortality was 5.6%, 5.6%, 5.5%, and 5.7% across quartiles 1 to 4, respectively (P‐trend=0.30). However, we observed a statistically significant trend toward reduced discharge to nursing facilities among survivors from high‐income SES quartiles compared with those from low‐income SES quartiles (P‐trend<0.001). There was no evidence of interaction between race and the SES categories for either of these study outcomes. Sensitivity analysis was performed by comparing the study outcomes between the study groups after removing all patients who presented as a “transfer” from another healthcare facility. There was no significant change in the trend of the incidence of the study outcomes across the SES quartiles with this analysis (Figure 2).
Figure 1.
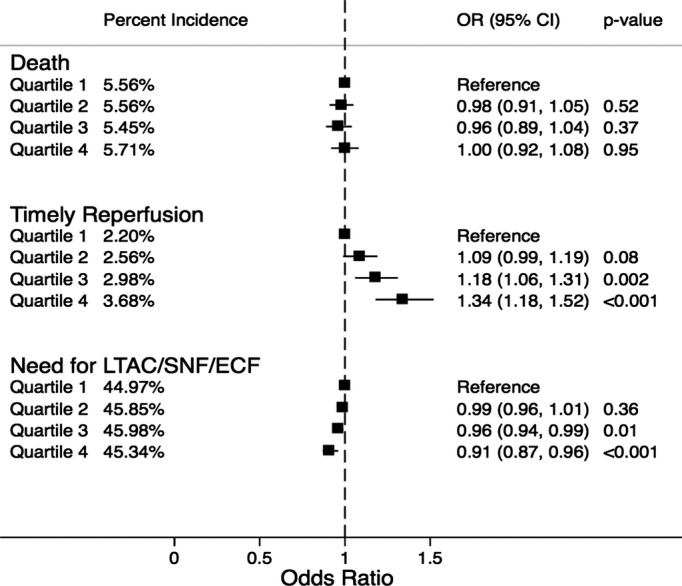
Percent incidence and adjusted odds ratio for in‐hospital mortality, timely reperfusion, and need for discharge to long‐term acute care or subacute nursing or extended care facilities, stratified by ZIP code–based socioeconomic quartiles. All quartiles were based on median household income of the respective ZIP code. All comparisons were drawn with reference to the lowest quartile. Quartile 1, $1 to $37 999; quartile 2, $38 000 to $47 999; quartile 3, $48 000 to $62 999; quartile 4, ≥$63 000. ECF indicates extended care facility; LTAC, long‐term acute care; OR, odds ratio; SNF, subacute nursing facility.
Figure 2.
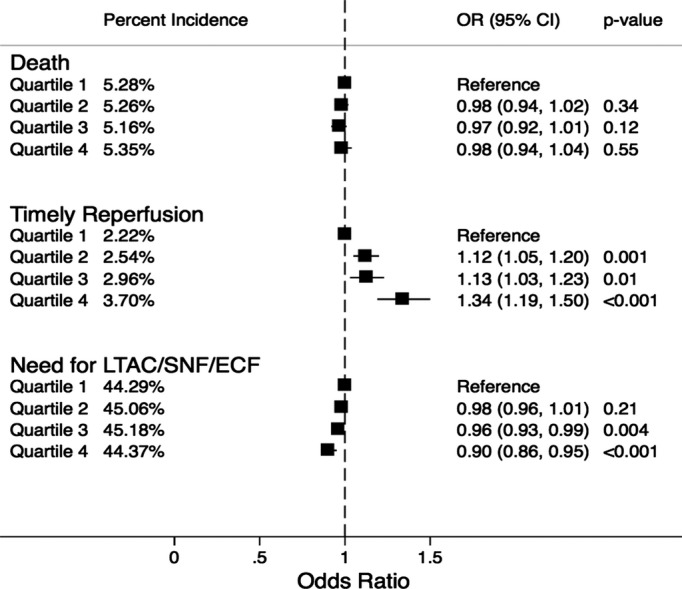
Percent incidence and adjusted odds ratio for in‐hospital death, timely reperfusion, and need for discharge to long‐term acute care or subacute nursing or extended care facilities, stratified by ZIP code–based socioeconomic quartiles after eliminating all patients who were transferred from an outside healthcare facility. All quartiles were based on median household income of the respective ZIP code. All comparisons were drawn with reference to the lowest quartile. Quartile 1, $1 to $37 999; quartile 2, $38 000 to $47 999; quartile 3, $48 000 to $62 999; quartile 4, ≥$63 000. ECF indicates extended care facility; LTAC, long‐term acute care; OR, odds ratio; SNF, subacute nursing facility.
The mean (SD) cost of hospitalization was $11 672 ($14 433), $11 666 ($14 361), $12 579 ($14 970), and $14 195 ($16 930) among SES quartiles 1 to 4, respectively. After adjustment for baseline demographic and clinical characteristics including primary payment source, the adjusted costs of hospitalization of patients from SES quartiles 2, 3, and 4 were higher than the cost of hospitalization of patients from SES quartile 1 (P≤0.001 for all comparisons). Compared with quartile 1, mean adjusted cost of hospitalization for patients from quartile 4 was higher by $2577 (95% CI: $1930 to $3225). Similarly, the mean adjusted cost of hospitalization for patients from quartiles 2 and 3 were higher by $621 (95% CI: $329 to $913) and $1238 (95% CI: $808 to $1667) compared with the costs of hospitalization of patients from SES quartile 1.
The mean (SE) LOS in the lowest‐income quartile was 5.75 (0.05) days, which was higher compared with other SES quartiles (P<0.001 compared with any other quartile). The mean (SE) LOS among quartiles 2, 3, and 4 was similar at 5.30 (0.3) days, 5.25 (0.04) days, and 5.31 (0.06) days, respectively. The proportion of patients requiring LOS ≥5 days in quartile 1 was 45.5%, which was higher than the corresponding proportions in other quartiles (41.8%, 41.4%, and 41.7% in quartiles 2, 3, and 4, respectively). Other resource use measures are demonstrated in Table 2. Compared with the lowest‐income quartile, there was a higher use of echocardiography, invasive angiography, and operative procedures on the head/neck region, including carotid endarterectomy in the highest‐income quartile (Table 2).
Table 2.
Percent Incidence and Odds Ratio for Resource Utilization Variables According to ZIP Code–Based Socioeconomic Quartiles
| ZIP Code Quartile | N | Percent Incidence | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Length of stay ≥5 d | ||||
| Quartile 1 | 230 006 | 45.45 | Reference | |
| Quartile 2 | 206 506 | 41.78 | 0.86 (0.84 to 0.89) | <0.001 |
| Quartile 3 | 182 985 | 41.41 | 0.85 (0.82 to 0.88) | <0.001 |
| Quartile 4 | 156 353 | 41.70 | 0.83 (0.81 to 0.86) | <0.001 |
| Echocardiogram use | ||||
| Quartile 1 | 230 028 | 10.29 | Reference | |
| Quartile 2 | 206 523 | 10.74 | 1.05 (0.97 to 1.14) | 0.24 |
| Quartile 3 | 182 991 | 11.38 | 1.12 (1.01 to 1.25) | 0.03 |
| Quartile 4 | 156 363 | 14.26 | 1.47 (1.16 to 1.84) | 0.001 |
| Invasive cerebral angiography | ||||
| Quartile 1 | 230 028 | 7.26 | Reference | |
| Quartile 2 | 206 523 | 6.91 | 0.95 (0.83 to 1.08) | 0.42 |
| Quartile 3 | 182 991 | 7.86 | 1.10 (0.95 to 1.28) | 0.20 |
| Quartile 4 | 156 363 | 8.88 | 1.26 (1.04 to 1.53) | 0.02 |
| Carotid endarterectomy | ||||
| Quartile 1 | 230 028 | 1.02 | Reference | |
| Quartile 2 | 206 523 | 1.29 | 1.27 (1.19 to 1.36) | <0.001 |
| Quartile 3 | 182 991 | 1.37 | 1.35 (1.25 to 1.46) | <0.001 |
| Quartile 4 | 156 363 | 1.26 | 1.25 (1.15 to 1.36) | <0.001 |
| Any operating room procedure on head/neck region including carotid endarterectomy | ||||
| Quartile 1 | 230 028 | 1.70 | Reference | |
| Quartile 2 | 206 523 | 2.02 | 1.19 (0.11 to 1.28) | <0.001 |
| Quartile 3 | 182 991 | 2.22 | 1.32 (1.21 to 1.44) | <0.001 |
| Quartile 4 | 156 363 | 2.20 | 1.31 (1.18 to 1.46) | <0.001 |
All quartiles were based on the median household income of the respective ZIP code. All comparisons were drawn with reference to the lowest‐income quartile. Quartile 1, $1 to $38 999; quartile 2, $39 000 to $47 999; quartile 3, $48 000 to $63 000; quartile 4, ≥$63 000. Quartile 1 reflects the lowest‐income quartile, and quartile 4 reflects the highest‐income quartile.
Subgroup analyses demonstrating the differential impact of sex, race, and age on in‐hospital death, reperfusion on first admission day, and need for discharge to nursing facilities are shown in Tables 3 through 5, respectively. There was no significant impact of sex or race on the incidence of in‐hospital mortality across the SES quartiles (Table 3). However, there was a differential impact of age on in‐hospital mortality rate among patients admitted with acute ischemic stroke. Although there was no significant difference in in‐hospital mortality across the SES quartiles in elderly patients (aged ≥75 years), we observed a significant reduction in in‐hospital mortality across the SES quartiles in younger patients (aged <75 years) (Table 3). With respect to reperfusion on first admission day, there was a progressive increase in the incidence and adjusted odds of reperfusion administration on first admission day across the SES quartiles in each subgroup strata of age, race, and sex (Table 4). With respect to discharge, there was a progressive decrease in the adjusted odds of discharge to nursing facilities across the SES quartiles in each subgroup strata of age, race, and sex (Table 5).
Table 3.
Percent Incidence and Odds Ratio for In‐Hospital Death According to ZIP Code–Based Socioeconomic Quartiles Stratified by Sex, Race, or Age
| ZIP Code Quartile | N | Percent Incidence | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Males | ||||
| Quartile 1 | 104 223 | 5.18 | Reference | |
| Quartile 2 | 94 733 | 5.24 | 0.99 (0.94 to 1.04) | 0.70 |
| Quartile 3 | 84 603 | 4.98 | 0.95 (0.89 to 1.02) | 0.11 |
| Quartile 4 | 72 967 | 5.09 | 0.96 (0.90 to 1.02) | 0.19 |
| Females | ||||
| Quartile 1 | 125 092 | 5.87 | Reference | |
| Quartile 2 | 111 304 | 5.84 | 0.97 (0.92 to 1.01) | 0.17 |
| Quartile 3 | 97 991 | 5.85 | 0.96 (0.91 to 1.01) | 0.11 |
| Quartile 4 | 82 994 | 6.25 | 0.99 (0.94 to 1.05) | 0.86 |
| Whites | ||||
| Quartile 1 | 103 730 | 6.20 | Reference | |
| Quartile 2 | 117 463 | 5.89 | 0.97 (0.93 to 1.01) | 0.18 |
| Quartile 3 | 109 809 | 5.65 | 0.96 (0.91 to 1.01) | 0.11 |
| Quartile 4 | 104 090 | 6.01 | 0.97 (0.91 to 1.02) | 0.23 |
| Nonwhites | ||||
| Quartile 1 | 78 500 | 4.62 | Reference | |
| Quartile 2 | 39 332 | 4.50 | 1.01 (0.95 to 1.08) | 0.76 |
| Quartile 3 | 33 686 | 4.56 | 1.03 (0.96 to 1.11) | 0.39 |
| Quartile 4 | 26 422 | 4.78 | 1.06 (0.97 to 1.16) | 0.17 |
| Age ≥75 y | ||||
| Quartile 1 | 98 454 | 7.90 | Reference | |
| Quartile 2 | 99 769 | 7.63 | 0.99 (0.95 to 1.04) | 0.75 |
| Quartile 3 | 92 273 | 7.38 | 0.99 (0.94 to 1.04) | 0.70 |
| Quartile 4 | 84 970 | 7.72 | 1.06 (0.97 to 1.15) | 0.21 |
| Age <75 y | ||||
| Quartile 1 | 130 899 | 3.80 | Reference | |
| Quartile 2 | 106 302 | 3.63 | 0.98 (0.92 to 1.03) | 0.41 |
| Quartile 3 | 90 343 | 3.47 | 0.92 (0.86 to 0.98) | 0.01 |
| Quartile 4 | 71 034 | 3.30 | 0.89 (0.83 to 0.96) | 0.002 |
All quartiles were based on the median household income of the respective ZIP code. All comparisons were drawn with reference to the lowest‐income quartile. All analyses have been adjusted for age, sex, race, 29 Elixhauser comorbidities, and other relevant comorbidities including smoking, dyslipidemia, primary payor, year of admission, and hospital characteristics. Quartile 1, $1 to $38 999; quartile 2, $39 000 to $47 999; quartile 3, $48 000 to $63 000; quartile 4, ≥$63 000. Quartile 1 reflects the lowest‐income quartile, and quartile 4 reflects the highest‐income quartile.
Table 5.
Percent Incidence and Odds Ratio for Need for Nursing Facilities Including Long‐Term Acute Care/Subacute Nursing/Extended Care Facility Among Hospital Survivors According to ZIP Code–Based Socioeconomic Quartiles Stratified by Sex, Race, or Age
| ZIP Code Quartile | N | Percent Incidence | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Males | ||||
| Quartile 1 | 98 827 | 41.02 | Reference | |
| Quartile 2 | 89 772 | 40.85 | 0.97 (0.94 to 0.99) | 0.028 |
| Quartile 3 | 80 387 | 40.56 | 0.94 (0.91 to 0.97) | <0.001 |
| Quartile 4 | 69 254 | 39.35 | 0.88 (0.83 to 0.93) | <0.001 |
| Females | ||||
| Quartile 1 | 117 746 | 48.30 | Reference | |
| Quartile 2 | 104 804 | 50.14 | 1.01 (0.98 to 1.04) | 0.54 |
| Quartile 3 | 92 259 | 50.71 | 0.99 (0.96 to 1.02) | 0.41 |
| Quartile 4 | 77 804 | 50.71 | 0.94 (0.89 to 0.99) | 0.038 |
| Whites | ||||
| Quartile 1 | 97 299 | 47.61 | Reference | |
| Quartile 2 | 110 541 | 46.98 | 0.96 (0.94 to 0.99) | 0.009 |
| Quartile 3 | 103 601 | 46.92 | 0.94 (0.91 to 0.97) | <0.001 |
| Quartile 4 | 97 832 | 46.54 | 0.88 (0.84 to 0.93) | <0.001 |
| Nonwhites | ||||
| Quartile 1 | 74 877 | 41.90 | Reference | |
| Quartile 2 | 37 562 | 41.33 | 1.00 (0.96 to 1.04) | 0.94 |
| Quartile 3 | 32 149 | 41.10 | 0.96 (0.91 to 0.99) | 0.042 |
| Quartile 4 | 25 158 | 40.63 | 0.92 (0.85 to 0.98) | 0.038 |
| Age ≥75 y | ||||
| Quartile 1 | 90 681 | 58.98 | Reference | |
| Quartile 2 | 92 160 | 59.62 | 1.01 (0.98 to 1.04) | 0.61 |
| Quartile 3 | 85 461 | 59.43 | 0.99 (0.95 to 1.02) | 0.39 |
| Quartile 4 | 78 412 | 57.98 | 0.93 (0.88 to 0.97) | 0.004 |
| Age <75 y | ||||
| Quartile 1 | 125 930 | 34.89 | Reference | |
| Quartile 2 | 102 448 | 33.46 | 0.97 (0.94 to 0.99) | 0.044 |
| Quartile 3 | 87 205 | 32.80 | 0.94 (0.91 to 0.98) | 0.001 |
| Quartile 4 | 68 688 | 30.93 | 0.90 (0.84 to 0.95) | 0.001 |
All quartiles were based on the median household income of the respective ZIP code. All comparisons were drawn with reference to the lowest‐income quartile. All analyses have been adjusted for age, sex, race, 29 Elixhauser comorbidities, and other relevant comorbidities including smoking, dyslipidemia, primary payor, year of admission, and hospital characteristics. Quartile 1, $1 to $38 999; quartile 2, $39 000 to $47 999; quartile 3, $48 000 to $63 000; quartile 4, ≥$63 000. Quartile 1 reflects the lowest‐income quartile, and quartile 4 reflects the highest‐income quartile.
Table 4.
Percent Incidence and Odds Ratio for Reperfusion Therapy Administered on First Admission Day According to ZIP Code–Based Socioeconomic Quartiles Stratified by Sex, Race, or Age
| ZIP Code Quartile | N | Percent Incidence | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Males | ||||
| Quartile 1 | 104 533 | 2.37 | Reference | |
| Quartile 2 | 94 913 | 2.81 | 1.17 (1.07 to 1.28) | <0.001 |
| Quartile 3 | 84 769 | 3.27 | 1.21 (1.10 to 1.33) | <0.001 |
| Quartile 4 | 73 121 | 3.98 | 1.43 (1.27 to 1.62) | <0.001 |
| Females | ||||
| Quartile 1 | 125 438 | 2.07 | Reference | |
| Quartile 2 | 111 562 | 2.35 | 1.11 (1.02 to 1.21) | 0.02 |
| Quartile 3 | 98 182 | 2.74 | 1.11 (0.99 to 1.23) | 0.06 |
| Quartile 4 | 83 176 | 3.42 | 1.31 (1.15 to 1.49) | <0.001 |
| Whites | ||||
| Quartile 1 | 104 038 | 2.51 | Reference | |
| Quartile 2 | 117 740 | 3.00 | 1.14 (1.05 to 1.23) | 0.002 |
| Quartile 3 | 110 071 | 3.40 | 1.17 (1.06 to 1.29) | 0.002 |
| Quartile 4 | 104 357 | 4.02 | 1.35 (1.19 to 1.53) | <0.001 |
| Nonwhites | ||||
| Quartile 1 | 78 805 | 2.53 | Reference | |
| Quartile 2 | 39 446 | 2.69 | 1.17 (1.04 to 1.31) | 0.007 |
| Quartile 3 | 33 741 | 2.91 | 1.15 (1.00 to 1.33) | 0.054 |
| Quartile 4 | 26 473 | 3.66 | 1.51 (1.26 to 1.82) | <0.001 |
| Age ≥75 y | ||||
| Quartile 1 | 98 749 | 1.85 | Reference | |
| Quartile 2 | 99 986 | 2.19 | 1.14 (1.03 to 1.25) | 0.009 |
| Quartile 3 | 92 447 | 2.61 | 1.14 (1.01 to 1.28) | 0.03 |
| Quartile 4 | 85 151 | 3.23 | 1.34 (1.17 to 1.54) | <0.001 |
| Age <75 y | ||||
| Quartile 1 | 131 257 | 2.47 | Reference | |
| Quartile 2 | 106 519 | 2.91 | 1.13 (1.05 to 1.23) | 0.002 |
| Quartile 3 | 90 525 | 3.36 | 1.16 (1.05 to 1.28) | 0.003 |
| Quartile 4 | 71 183 | 4.21 | 1.37 (1.21 to 1.54) | <0.001 |
All quartiles were based on the median household income of the respective ZIP code. All comparisons were drawn with reference to the lowest‐income quartile. All analyses have been adjusted for age, sex, race, 29 Elixhauser comorbidities, and other relevant comorbidities including smoking, dyslipidemia, primary payor, year of admission, and hospital characteristics. Quartile 1, $1 to $38 999; quartile 2, $39 000 to $47 999; quartile 3, $48 000 to $63 000; quartile 4, ≥$63 000. Quartile 1 reflects the lowest‐income quartile, and quartile 4 reflects the highest‐income quartile.
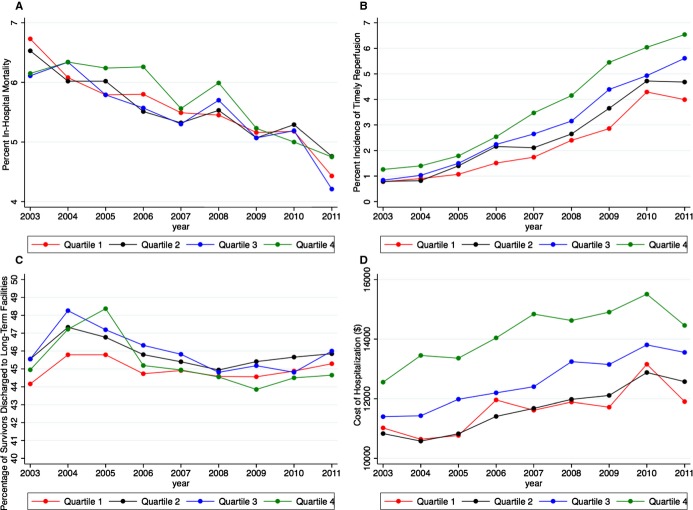
Figure 3 demonstrates the variation in the trend of in‐hospital mortality, reperfusion on the first admission day, and the cost of hospitalization among patients admitted with acute ischemic stroke during the study. Although there has been a considerable reduction in in‐hospital mortality over the years in all SES categories, there was no significant difference in in‐hospital mortality evident between the socioeconomic strata (Figure 3A). On the contrary, the disparity in the incidence of reperfusion on the first admission day across the SES categories has appeared to increase during the study duration (Figure 3C). Furthermore, there has been a steady increase in the cost of hospitalization in all 4 SES categories during the study, with the costs remaining substantially higher in the high‐income SES quartiles compared with the low‐income SES quartiles across all included years (Figure 3D).
Figure 3.
Trends demonstrating the incidence of study outcomes across the 4 socioeconomic quartiles across the entire study duration (2003–2011). A, Trend of in‐hospital mortality. B, Trend of timely reperfusion. C, Trend of discharge to long‐term acute care/subacute nursing/extended care facility. D, Trend of the cost of hospitalization. Quartile 1, $1 to $38 999; quartile 2, $39 000 to $47 999; quartile 3, $48 000 to $63 000; quartile 4, ≥$63 000.
Discussion
The current study sought to evaluate the impact of residential ZIP code–based classification of SES on adverse events following acute ischemic stroke by using a large representative nationwide sample in the United States. We observed a progressive increase in the incidence of reperfusion on the first admission day with an improvement in the median household income associated with the patient's residential ZIP code. This was evident in both sexes (males and females), both racial strata (whites and nonwhites), and both age group (<75 years, ≥75 years) strata. Although we did not observe a difference in in‐hospital mortality across the SES quartiles in the overall cohort, there was a significant trend toward reduced in‐hospital mortality across the SES quartiles in younger patients (aged <75 years). In addition, we observed a trend demonstrating a progressive increase in the unadjusted and adjusted costs of hospitalization across the SES quartiles. Further, we noted a significantly longer hospital stay among lowest‐income quartile compared with other SES quartiles. However, despite a longer LOS among the lowest‐income quartile patients, there was a lower use of echocardiography, invasive angiography, and operative interventions among these patients compared with the high‐income quartile patients. Whether this represents an underuse of resources for patients from lower‐income SES ZIP codes or an overuse of resources for patients from higher‐income SES ZIP codes is not readily apparent from this analysis. However, our analysis has demonstrated a disparity in resource use for acute ischemic stroke patients depending on the residential ZIP code.
The differences in SES have been consistently associated with variations in morbidity and mortality related to cerebrovascular disease.7,9 Individuals residing in neighborhoods with a lower SES have a higher prevalence of traditional risk factors such as hypertension, diabetes, obesity, and smoking, which might account for the observed association between the contextual factors and outcomes after acute ischemic stroke. Similar trends have been noted in our analysis. However, the differences in traditional risk factors are unlikely to be the sole explanation for differences in clinical outcomes. The differences in several observed clinical outcomes persist between the SES quartiles persist despite adjustment for baseline risk factors, suggesting that other factors in healthcare access and delivery likely play a role. Lower SES has been demonstrated to limit access to medical care and to bias these patients to present to smaller, low‐volume hospitals with lower use of evidence‐based therapies.6–7,9 Although we observed no difference in overall mortality, the reduced mortality observed with increasing SES in those aged <75 years accompanied by the lower rate of disability makes this an important clinical finding. It is possible that unmeasured prehospital variables clustered by SES may account for the differences noted. These include timely recognition of stroke symptoms, performance characteristics of the local emergency medical services systems, inappropriate triage and transport to nonreperfusion hospitals, and the availability of postdischarge support at home to avoid the need for nursing facilities.
There is a large body of literature that has evaluated the existence and impact of racial disparities on outcomes after acute ischemic stroke.29–32 Although we found a significant higher proportion of nonwhites in the lower socioeconomic strata, “socioeconomic disparity” and “racial disparity” are hardly interchangeable terms. Hence, although it is convenient to label patients by race, factors that relate to SES, like income, education, housing, and social awareness, are probably more important in health‐related outcomes. Socioeconomic position has been speculated to be a greater impediment to optimal health rather than “biologically implausible surrogates of race and sex.”33 Despite a breadth of evidence spanning the relationship of racial disparities with adverse outcomes, relatively fewer studies have exclusively evaluated the role of SES on outcomes after acute ischemic stroke. Few studies have demonstrated that short‐term survival at 30 days might not be impacted by SES.7,34 This finding was present in our analysis, too. It has been suggested that stroke severity, rather than SES, is the primary predictor of short‐term survival after acute ischemic stroke.34 Although predictive of long‐term mortality, stroke severity is of less importance in predicting survival at 1 year or longer. Although there was no direct impact of SES on inpatient mortality in our study, we did observe a significant association between SES and the need for discharge to nursing facilities. This disparity might suggest an indirect impact of SES on the severity of stroke at presentation, which in turn might impact medium‐ or long‐term mortality.
During the past decade, remarkable progress has been made in the delivery of optimal reperfusion for patients with acute ischemic stroke. However, despite these improvements, the absolute numbers of patients who benefit from these treatment modalities remain small. We believe that socioeconomic parameters have a small impact on the door‐to‐needle times after a patient presents to a healthcare facility. However, a greater degree of impact of SES probably occurs on the duration between symptom onset and first medical contact. Several socioeconomic and sociocultural factors, including education, access to healthcare resources, income, and awareness, might play important roles in determining the overall total ischemic time following acute ischemic stroke, thereby directly affecting the outcome. The impact of surrounding environment becomes highly significant in this context. Further, one could argue that the impact of environment, substituted by an aggregate ZIP code income, might provide a greater degree of insight into society–health dynamics and interactions than does individual household income.
One of the major findings of our study was a disparity in the healthcare resource use among different SES quartiles. Many prior studies have demonstrated significant socioeconomic disparities in the use of thrombolysis for the treatment of acute ischemic stroke.5,8,31–32,35 In addition, a recent study has demonstrated significant disparities exist in the use of mechanical thrombectomy across the various socioeconomic strata,6 partially attributable to lack of timely access to centers offering this treatment. However, the trends in these disparities during the past decade remain poorly understood. Our study clearly demonstrates that despite a considerable improvement in reperfusion rates across all categories, the apparent disparity in timely treatment and the cost of hospitalization continue to increase across the socioeconomic strata.
Limitations
Our study has a several limitations. First, NIS is an administrative database, which may be subject to errors in coding of diseases or procedures. Because the unit of analysis in the NIS database is “unique admission” rather than “unique patient,” it is possible that one patient might have been represented more than once, in case of repeat admission for recurrent ischemic stroke. Although the ICD codes 99.10 and V45.88 have been shown to be highly specific for ascertaining thrombolysis, their sensitivity might be low, leading to underestimation of the true incidence of reperfusion.31,36 However, we believe that this is unlikely to introduce a bias in our study, as this underestimation should apply equally to all socioeconomic strata. Second, this is a retrospective observational study, which may be subject to traditional biases of observational studies like selection bias. However, these limitations might be partially compensated due to the large size of the NIS database and a uniform representation of all regions of the United States. Third, the definition of “timely reperfusion” included all patients who underwent reperfusion on “day 0” of admission to the hospital, due to lack of availability of hourly data. This definition might lend itself to slight overinclusion of patients who may not have undergone reperfusion by strict stroke chain of survival standards.37 Fourth, decision to administer reperfusion therapy depends on multiple clinical variables such as time from stroke onset to presentation and stroke severity, which were not available in the database. In addition, adverse outcomes after acute stroke may be affected by numerous variables, including stroke severity, symptom awareness, and prehospital stroke care delivery, along with poststroke variables like risk factor modification and treatment adherence after hospital discharge, which were not available consistently in the NIS database.
A further limitation might result from the fact that we used median household income of the entire ZIP code to “impute” the SES of each patient. The capability of an individual measure like the median household income of the residential ZIP code to directly relate to the SES of each patient may be somewhat limited. However, the inaccuracy resulting from the misclassification of personal SES based on SES of the surrounding neighborhood (“ecologic fallacy”) may be completely offset by the lack of occurrence of an “individualistic fallacy,” whereby there is an incorrect assumption that the health of an individual subject is not affected by the neighborhood in which they reside.38 Despite this, it is certainly possible that “area‐level” measures of SES might not always correlate with individual SES and might result in incorrect conclusions. A composite measure of SES that includes several variables like directly measured household income, education, race, and residential ZIP code might provide incremental information regarding individual SES. However, several characteristics that may be useful in defining composite measures of SES, like education and household income, were not available in the administrative database of the NIS.
Conclusions
In patients presenting with acute ischemic stroke, there was a progressive increase in the incidence of reperfusion administration on the first admission day with increasing median household income of the residential ZIP code. In addition, there was a progressive increase in the unadjusted and adjusted cost of hospitalization across the SES quartiles despite a paradoxically longer hospital stays among the lowest‐income quartile patients. This might be partially attributable to a lower use of procedures such as echocardiography, invasive angiography and operative interventions among patients from lowest‐income quartile, compared with highest‐income quartile patients. Despite these disparities, we did not observe a significant difference in in‐hospital mortality across the SES quartiles.
Acknowledgments
The author would like to acknowledge the help of Kathryn Brock in editing the manuscript.
Disclosures
None.
References
- Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009; 8:345-354. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Shibuya K, Boschi‐Pinto C, Lopez AD, Murray CJ. Global and regional estimates of cancer mortality and incidence by site: I. Application of regional cancer survival model to estimate cancer mortality distribution by site. BMC Cancer. 2002; 2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007; 6:182-187. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Garg A, Parashar A, Jaber WA, Menon V. Outcomes and resource utilization in ST‐elevation myocardial infarction in the United States: evidence for socioeconomic disparities. J Am Heart Assoc. 2014; 3:e00105710.1161/JAHA.114.001057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addo J, Ayerbe L, Mohan KM, Crichton S, Sheldenkar A, Chen R, Wolfe CD, McKevitt C. Socioeconomic status and stroke: an updated review. Stroke. 2012; 43:1186-1191. [DOI] [PubMed] [Google Scholar]
- Brinjikji W, Rabinstein AA, Cloft HJ. Socioeconomic disparities in the utilization of mechanical thrombectomy for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014; 23:979-984. [DOI] [PubMed] [Google Scholar]
- Brown AF, Liang LJ, Vassar SD, Merkin SS, Longstreth WT, Jr, Ovbiagele B, Yan T, Escarce JJ. Neighborhood socioeconomic disadvantage and mortality after stroke. Neurology. 2013; 80:520-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz‐Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, Howard G, Leira EC, Morgenstern LB, Ovbiagele B, Peterson E, Rosamond W, Trimble B, Valderrama ALAmerican Heart Association Stroke C, Council on Cardiovascular N, Council on E, Prevention, Council on Quality of C and Outcomes R. Racial‐ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:2091-2116. [DOI] [PubMed] [Google Scholar]
- Kapral MK, Fang J, Chan C, Alter DA, Bronskill SE, Hill MD, Manuel DG, Tu JV, Anderson GM. Neighborhood income and stroke care and outcomes. Neurology. 2012; 79:1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BE, Rafferty AP, Lyon‐Callo S, Fussman C, Reeves MJ. Knowledge of tissue plasminogen activator for acute stroke among Michigan adults. Stroke. 2009; 40:2564-2567. [DOI] [PubMed] [Google Scholar]
- Sallar AM, Williams PB, Omishakin AM, Lloyd DP. Stroke prevention: awareness of risk factors for stroke among African American residents in the Mississippi delta region. J Natl Med Assoc. 2010; 102:84-94. [DOI] [PubMed] [Google Scholar]
- Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, Zhao X, Peterson E, Fonarow GC. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010; 121:1492-1501. [DOI] [PubMed] [Google Scholar]
- Aslanyan S, Weir CJ, Lees KR, Reid JL, McInnes GT. Effect of area‐based deprivation on the severity, subtype, and outcome of ischemic stroke. Stroke. 2003; 34:2623-2628. [DOI] [PubMed] [Google Scholar]
- Brown P, Guy M, Broad J. Individual socio‐economic status, community socio‐economic status and stroke in New Zealand: a case control study. Soc Sci Med. 2005; 61:1174-1188. [DOI] [PubMed] [Google Scholar]
- Engstrom G, Jerntorp I, Pessah‐Rasmussen H, Hedblad B, Berglund G, Janzon L. Geographic distribution of stroke incidence within an urban population: relations to socioeconomic circumstances and prevalence of cardiovascular risk factors. Stroke. 2001; 32:1098-1103. [DOI] [PubMed] [Google Scholar]
- Lisabeth LD, Diez Roux AV, Escobar JD, Smith MA, Morgenstern LB. Neighborhood environment and risk of ischemic stroke: the brain attack surveillance in Corpus Christi (BASIC) Project. Am J Epidemiol. 2007; 165:279-287. [DOI] [PubMed] [Google Scholar]
- Morgenstern LB, Escobar JD, Sanchez BN, Hughes R, Zuniga BG, Garcia N, Lisabeth LD. Fast food and neighborhood stroke risk. Ann Neurol. 2009; 66:165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrift AG, Dewey HM, Sturm JW, Paul SL, Gilligan AK, Srikanth VK, Macdonell RA, McNeil JJ, Macleod MR, Donnan GA. Greater incidence of both fatal and nonfatal strokes in disadvantaged areas: the Northeast Melbourne Stroke Incidence Study. Stroke. 2006; 37:877-882. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Bound J, Niedert L. On the validity of using census geocode characteristics to proxy economic status. Population Studies Center. Ann Arbor, MI: University of Michigan; 1993. (Research reports no. 93x2010269). [Google Scholar]
- Geronimus AT, Bound J, Niedert L. On the validity of using census geocode characteristics to proxy individual socioeconomic characteristics. Technical working paper 189. Cambridge, MA: National Bureau of Economic Research; 1995. [Google Scholar]
- Mcbean AM, Hebert P. Comparison of income information in the 1990 census with information in the Medicare current beneficiary survey. Baltimore, MD: Health Care Financing Administration; 1995. (HCFA contract no. HCFAx201095x20100265). [Google Scholar]
- Carr‐Hill R, Rice N. Is enumeration district level an improvement on ward level analysis in studies of deprivation and health? J Epidemiol Community Health. 1995; 49suppl 2:S28-S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census‐based methodology. Am J Public Health. 1992; 82:703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Palmer L. Clinical Classification Software (CCS). US Agency for Healthcare Research and Quality; 2014. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/ccs.ccs.jsp. Accessed November 21, 2014 [Google Scholar]
- Healthcare Cost and Utilization Project (HCUP). HCUP Comorbidity Software. 2013Rockville, MD: Agency for Healthcare Research and Quality; 2013 [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998; 36:8-27. [DOI] [PubMed] [Google Scholar]
- Consumer price index‐ guide to CPI data. United States Department of Labor. Bureau of Labor Statisitcs; http://www.bls.gov/cpi/cpifact8.htm. Accessed May 22, 2014. [Google Scholar]
- Cohen J. Statistical Power Analysis for Behavioral Sciences. 1988New Jersey: Lawrence Erlbaum Associates, Inc; 1988. 283-286. [Google Scholar]
- Bhattacharya P, Mada F, Salowich‐Palm L, Hinton S, Millis S, Watson SR, Chaturvedi S, Rajamani K. Are racial disparities in stroke care still prevalent in certified stroke centers? J Stroke Cerebrovasc Dis. 2013; 22:383-388. [DOI] [PubMed] [Google Scholar]
- Jaja BN, Saposnik G, Nisenbaum R, Schweizer TA, Reddy D, Thorpe KE, Macdonald RL. Effect of socioeconomic status on inpatient mortality and use of postacute care after subarachnoid hemorrhage. Stroke. 2013; 44:2842-2847. [DOI] [PubMed] [Google Scholar]
- Johnston SC, Fung LH, Gillum LA, Smith WS, Brass LM, Lichtman JH, Brown AN. Utilization of intravenous tissue‐type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity. Stroke. 2001; 32:1061-1068. [DOI] [PubMed] [Google Scholar]
- Nasr DM, Brinjikji W, Cloft HJ, Rabinstein AA. Racial and ethnic disparities in the use of intravenous recombinant tissue plasminogen activator and outcomes for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013; 22:154-160. [DOI] [PubMed] [Google Scholar]
- Koch CG, Li L, Kaplan GA, Wachterman J, Shishehbor MH, Sabik J, Blackstone EH. Socioeconomic position, not race, is linked to death after cardiac surgery. Circ Cardiovasc Qual Outcomes. 2010; 3:267-276. [DOI] [PubMed] [Google Scholar]
- Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke. 2009; 40:2068-2072. [DOI] [PubMed] [Google Scholar]
- Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the United States. J Hosp Med. 2010; 5:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, Harris‐Lane P, Siddiqi F, Kirmani JF. International classification of diseases and current procedural terminology codes underestimated thrombolytic use for ischemic stroke. J Clin Epidemiol. 2006; 59:856-858. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Cucchiara B, Adeoye O, Meurer W, Brice J, Chan YY, Gentile N, Hazinski MF. Part 11: adult stroke: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122:S818-S828. [DOI] [PubMed] [Google Scholar]
- Alter DA, Naylor CD, Austin P, Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med. 1999; 341:1359-1367. [DOI] [PubMed] [Google Scholar]