Abstract
Background
Up to 50% of penile squamous cell carcinomas (pSCC) develop in the context of high-risk human papillomavirus (HR-HPV) infection. Most of these tumours have been reported to show basaloid differentiation and overexpression of tumour suppressor protein p16INK4a. Whether HPV-triggered carcinogenesis in pSCC has an impact on tumour aggressiveness, however, is still subject to research.
Methods
In tissue specimens from 58 patients with surgically treated pSCC between 1995 and 2012, we performed p16INK4a immunohistochemistry and DNA extraction followed by HPV subtyping using a PCR-based approach. The results were correlated with histopathological and clinical parameters.
Results
90.4% of tumours were of conventional (keratinizing) subtype. HR-HPV DNA was detected in 29.3%, and a variety of p16INK4a staining patterns was observed in 58.6% of samples regardless of histologic subtype. Sensitivity of basaloid subtype to predict HR-HPV positivity was poor (11.8%). In contrast, sensitivity and specificity of p16INK4a staining to predict presence of HR-HPV DNA was 100% and 57%, respectively. By focussing on those samples with intense nuclear staining pattern for p16INK4a, specificity could be improved to 83%. Both expression of p16INK4a and presence of HR-HPV DNA, but not histologic grade, were inversely associated with pSCC tumour invasion (p = 0.01, p = 0.03, and p = 0.71). However, none of these correlated with nodal involvement or distant metastasis. In contrast to pathological tumour stage, the HR-HPV status, histologic grade, and p16INK4a positivity failed to predict cancer-specific survival.
Conclusions
Our results confirm intense nuclear positivity for p16INK4a, rather than histologic subtype, as a good predictor for presence of HR-HPV DNA in pSCC. HR-HPV / p16INK4a positivity, independent of histological tumour grade, indicates a less aggressive local behaviour; however, its value as an independent prognostic indicator remains to be determined. Since local invasion can be judged without p16INK4a/HPV-detection on microscopic evaluation, our study argues against routine testing in the setting of pSCC.
Keywords: Human papillomavirus, Invasion, Metastasis, p16INK4a, Penile cancer
Background
Squamous cell carcinoma of the penis (pSCC) is associated with high morbidity and mortality and causes severe psychological impact [1,2]. High-risk human papillomavirus (HR-HPV) DNA is detectable in 30-80% of pSCC surgical specimens [3]. In total, more than 17,000 cancer cases in European men per year are attributable to HPV, with more than 15,000 of them being specifically attributable to HR-HPV-16 or -18 [4]. The benefit of early vaccination of girls and boys using a quadrivalent HPV vaccine to prevent later occurrence of HPV-related diseases is a current subject of discussion [5]. Integration of HR-HPV DNA into the host cell genome leads to overexpression of viral oncoproteins E6 and E7 that exert a dysregulating effect on cell cycle control [6]; upregulation of tumour suppressor protein p16INK4a is understood as an attempt to stop uncontrolled cell proliferation in response to HPV infection [7]. P16INK4a immunohistochemistry is therefore used as a surrogate marker for high-risk HPV in cancers of the cervix uteri and the head and neck region [8,9]. In bladder cancer, however, we did not detect HPV DNA in 29 samples of urothelial carcinoma in situ despite diffuse intense confluent staining pattern for p16INK4a [10]. Chaux et al. reported that in pSCC, HPV-associated tumours are frequently composed of undifferentiated warty or basaloid cells that show a variable degree of koilocytic changes. The same group also noted that non-HPV tumours are composed of keratinizing differentiated squamous cells [11]. Concerning prognosis, HPV-driven squamous cell carcinomas of the head and neck region (HNSCCs) are associated with significantly improved progression-free survival and disease-free survival [12]. On the other hand, published data suggests a role for HPV-16 E6/E7 oncoproteins in the induction of epithelial-mesenchymal transition which may contribute to cell migration and metastasis [13,14]. Therefore, the impact of virus-associated tumourigenesis on tumour aggressiveness, metastatic potential, and patient prognosis in penile cancer is still unclear, since only a few studies have addressed this topic, and the results are inconsistent [15-17]. In the present study, we analysed pSCC tissue samples for expression of p16INK4a and presence of HPV DNA and correlated the results with tumour- and patient-specific characteristics as well as cancer-specific survival. We evaluated the value of histologic subtype and various p16INK4a staining patterns as indicators for the presence of HR-HPV DNA in tumour tissue, and showed for the first time that presence of HR-HPV DNA in pSCC is inversely associated with local tumour invasion. Furthermore, we analysed the impact of HPV-driven tumourigenesis on cancer-specific survival in pSCC patients.
Methods
Ethics statement
All specimens were surgically removed for therapeutic purposes and subsequent histologic examination and were thoroughly pseudonymized for the use in this study. Therefore, individual written informed consent was not mandatory. The study was approved by the University of Ulm ethics committee (Approval No. 331/2013).
Patient samples
Tissue samples from 58 patients were included in the study; for all patients, biological parameters and tumour characteristics were available. Patients underwent surgery for penile cancer between 1995 and 2012 at the Ulm University Medical Centre (n = 37) or at the Bundeswehrkrankenhaus Ulm (n = 21). All specimens had been submitted for routine histologic examination to the Institutes of Pathology of either the University of Ulm or the Bundeswehrkrankenhaus Ulm. Clinico-pathological characteristics are summarized in Table 1. TNM stages were determined according to the UICC classification of 2010 [18]. T stages pTis (carcinoma in situ) and pTa (papillary/verrucous carcinoma) were summarized as noninvasive, while pT1-pT4 stages were classified as invasive lesions. Patient and tumour characteristics obtained from the institutional databases included age, stage, and the presence of histologically verified regional lymph node involvement or distant metastasis, respectively. Of 35 patients (60.3% of the complete cohort; 23 HPV-negative and 12 HPV-positive cases), complete follow-up data was obtainable and reached up to 204 months (median/mean follow-up 15/31 months).
Table 1.
Clinico-pathologic sample characteristics
| No. of patients | 58 | ||
|---|---|---|---|
| No. of samples | 58 | ||
| Age (mean; range) | 64.5; 31-93 years | ||
| Tumour characteristics | |||
| Differentiation | Distant metastasis | ||
| keratinizing | 53 | Mx | 21 |
| basaloid | 5 | cM0 | 33 |
| Grading | pM+ | 4 | |
| Low-grade (G1-2) | 43 | ||
| High-grade (G3-4) | 15 | p16 INK4a IHC | |
| pT stage | negative | 24 | |
| pTis | 10 | positive | 34 |
| pTa | 2 | intense confluent | 24 |
| pT1 | 19 | focally scattered | 10 |
| pT2 | 20 | ||
| pT3 | 5 | ||
| pT4 | 2 | HPV subtyping | |
| Nodal involvement | HPV- | 40 | |
| Nx | 30 | HPV+ | 18 |
| pN0 | 15 | HPV-16 | 16 |
| pN+ | 13 | HPV-45 | 1 |
| HPV-6 | 1 | ||
pTis, carcinoma in situ; pTa, papillary/exophytic (noninvasive) carcinoma; pT1-pT4, depth of tumor invasion; Nx, lymph node status unknown; pN0, (verified) absence of lymph node metastases; pN+, (verified) presence of lymph node metastases; Mx, presence of distant metastases unknown; cM0, no clinical evidence for distant metastases; pM+, (verified) presence of distant metastases; IHC, immunohistochemistry; HPV, human papillomavirus.
Immunohistochemistry, image acquisition, and expression analysis
Immunohistochemistry for p16INK4a was performed on a BenchMark Autostainer (Ventana Medical Systems, Tucson, USA) according to the manufacturer’s protocol using a prediluted mouse monoclonal antibody (CINtec® p16 Histology, clone E6H4, Ventana Medical Systems, Tucson, USA). Microscopic slide evaluation/image acquisition was performed using a Leica DM6000B light microscope (Leica, Wetzlar, Germany) and the Diskus Mikroskopische Diskussion image acquisition software (Carl H. Hilgers, Königswinter, Germany). P16INK4a staining patterns were classified in the following categories: a) intense confluent or b) focally scattered nuclear and/or cytoplasmic staining pattern [19]. Histological subtype, tumour grade and p16INK4a staining patterns were confirmed by an experienced pathologist (PM).
Human papillomavirus (HPV) testing
HPV genotyping was performed as previously described [10]. In short, DNA was extracted from tumour-containing paraffin slides using the automated Maxwell® 16 FFPE Plus LEV DNA Purification Kit (Promega, Madison, USA) and amplified with biotinylated primers; PCR products were incubated with oligonucleotide-precoated strips using the HPV typing kit from AID diagnostics (Strassberg, Germany) according to the manufacturer’s protocol (Figure 1). The kit detects 15 different HPV genotypes (6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58 and 59).
Figure 1.
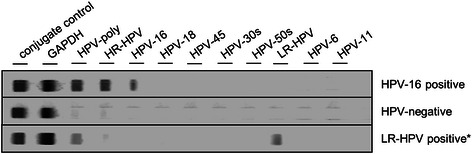
Representative results of HPV genotyping using a PCR-based approach. First lane shows a HPV-16-positive case, while in lane 2, no HPV DNA was detectable. Lane 3 shows detection of LR-HPV DNA that was later identified as HPV isoform 6. LR-HPV, low-risk human papillomavirus.
Statistical analysis
Correlations between dichotomous variables (histologic subtype, p16INK4a positivity, HPV status, presence/absence of tumour invasion, presence/absence of lymph node involvement or distant metastasis, tumour differentiation grade) were tested for significance using the Chi-Square/Fisher’s exact test (SPSS, IBM, Ehningen, Germany). The difference in disease-specific survival between low-grade/high-grade, p16INK4a+/p16INK4a- and HPV+/HPV- groups was assessed using LogRank test (SPSS, IBM, Ehningen, Germany). A two-tailed p-value of <0.05 was regarded as statistically significant.
Results
Clinico-pathologic sample characteristics
The mean (median) age of the 58 patients included in the study was 64.5 (64.0) years (IQR, 55.5-73.3 years, Table 1). 43/58 (74.1%) samples were histologically classified as low-grade (G1-2) tumours; 53/58 (91.4%) of the specimens showed keratinizing growth pattern with formation of keratin pearls (Figure 2C), while 5/58 (8.6%) tumours were of the basaloid subtype. Non-invasive tumour growth (pTis/pTa stages) was observed in 12/58 (20.7%) samples. Presence of lymph node or distant metastases was confirmed in pathological workup in 13 (22.4%) and 4 (6.9%) cases, respectively.
Figure 2.
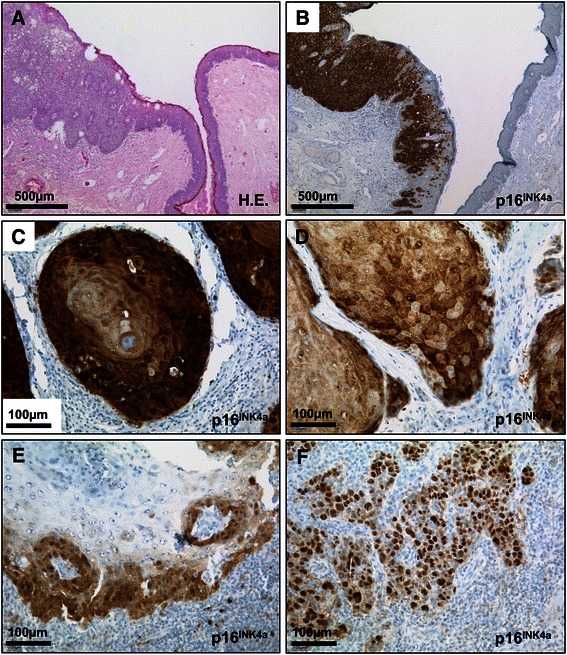
Representative microphotographs of pSCC specimens. A and B, penile squamous cell carcinoma in situ displaying strong, confluent expression of p16INK4a. Note ascending p16INK4a positivity in non-malignant epithelium adjacent to carcinoma. C-F, variety of p16INK4a staining patterns in pSCC: C, strong, confluent staining in invasive keratinizing carcinoma; D, diffuse and E, focally scattered positivity for p16INK4a; F, almost exclusively nuclear immunostaining. Scale bars as indicated; H.E., haematoxylin-eosin; HR-HPV, high-risk human papillomavirus; pSCC, penile squamous cell carcinoma.
P16INK4a Immunohistochemistry
Altogether, positive immunostaining for p16INK4a was observed in 34 (58.6%) tumour samples, with 30/53 (56.6%) tumours of the keratinizing subtype showing some degree of positivity. However, there was a wide variety in p16INK4a expression patterns (Figure 2): intense confluent staining pattern (Figure 2B and C) was detected in 24 of the 34 (70.6%) positive cases, while weak or focal scattered staining (Figure 2D and E) was observed in 10 (29.4%) samples. Among the cases with ubiquitous strong expression in all tumour cells, there was also a variety in cellular distribution of p16INK4a from almost exclusively nuclear (3 cases, Figure 2F) to intense cytoplasmic and nuclear positivity (22 cases, Figure 2B and C). There was no significant association between the observed p16INK4a staining pattern and the respective area of individual tumors (superficial/ exophytic versus invasive areas).
HPV genotyping
Genomic subtyping following DNA extraction confirmed presence of HPV DNA in 18 (31.0%) of 58 samples, 16 of which were classified as HPV-16 (Figure 1). One sample turned out to harbour HPV-45, while one case was positive for HPV-6 DNA (low risk HPV). Notably, PCR amplification of HPV-DNA was also successful in one case where the paraffin block dated back to 1995.
HPV status and clinic-pathological parameters
Basaloid histologic subtype of pSCC was not significantly associated with presence of HR-HPV (HPV-16 or HPV-45) in our study cohort (p = 0.62; Fisher’s exact test; Table 2). Although the specificity of the basaloid growth pattern for HR-HPV was 92.7%, since 28.3% of the keratinizing tumours were also found to be HR-HPV positive, sensitivity was only 11.8% (positive predictive value (PPV), 40%; Table 3). P16INK4a immunopositivity, irrespective of the observed staining pattern, was significantly associated with presence of HR-HPV DNA (p < 0.001; Fisher’s exact test; Table 2). However, the PPV of p16INK4a staining for presence of HR-HPV was only 52.9% (sensitivity, 100%; specificity, 60%). This predictor was markedly improved by focusing on cases with intense nuclear and confluent staining patterns in all tumour cells (PPV, 75%; specificity, 85%; Table 3). Histopathologic tumour grade was not significantly associated with presence of HPV DNA. HR-HPV positivity and nuclear p16INK4a staining, but not histologic differentiation grade, was significantly associated with non-invasive tumour growth (pTis/pTa stage; p = 0.03, p = 0.01, and p = 0.71). For verified nodal involvement or distant metastasis, there was no significant association with HPV status, p16INK4a positivity or grade of differentiation of the primary tumour (p = 0.22, p = 0.25, and p = 0.41; Fisher’s exact test).
Table 2.
HPV status and clinico-pathological parameters
| HR-HPV- | HR-HPV+ | p-value* | |
|---|---|---|---|
| Histopathologic subtype | |||
| keratinizing | 38 | 15 | |
| basaloid | 3 | 2 | 0.62 |
| Grading | |||
| Low-grade (G1-2) | 32 | 11 | |
| High-grade (G3-4) | 9 | 6 | 0.33 |
| Presence of tumour invasion | |||
| Non-invasive | 5 | 7 | |
| Invasive | 36 | 10 | 0.028 |
| Presence of metastasis | |||
| N0M0 | 10 | 5 | |
| N+ and/or M+ | 11 | 2 | 0.40 |
| p16 INK4a immunostaining | |||
| negative | 24 | 0 | |
| scattered | 10 | 0 | |
| intense nuclear/confluent | 7 | 17 | <0.001 |
*Fisher’s exact Test.
Table 3.
Test statistics for basaloid histologic subtype and p16 INK4a immunostaining to predict HR-HPV positivity in pSCC
| Test | ppV (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Basaloid histologic subtype | 40 | 11 | 95 |
| Any positivity for p16INK4a | 53 | 100 | 60 |
| Intense nuclear p16INK4a staining | 75 | 100 | 85 |
HPV status and cancer-specific survival
Follow-up data was obtainable for 35 patients and reached up to 204 months. No patient in the HPV-positive group, but 6 patients in the HPV-negative group died from the disease (Figure 3). However, this difference failed to reach significance in Kaplan-Meier analysis (p = 0.13; Log Rank test). In contrast to pathological tumour stage including all stages (p = 0.01), p16INK4a positivity and histologic differentiation grade (G1-2 vs. G3-4) were also not significantly associated with cancer-specific survival (p = 0.49 and p = 0.15, respectively).
Figure 3.
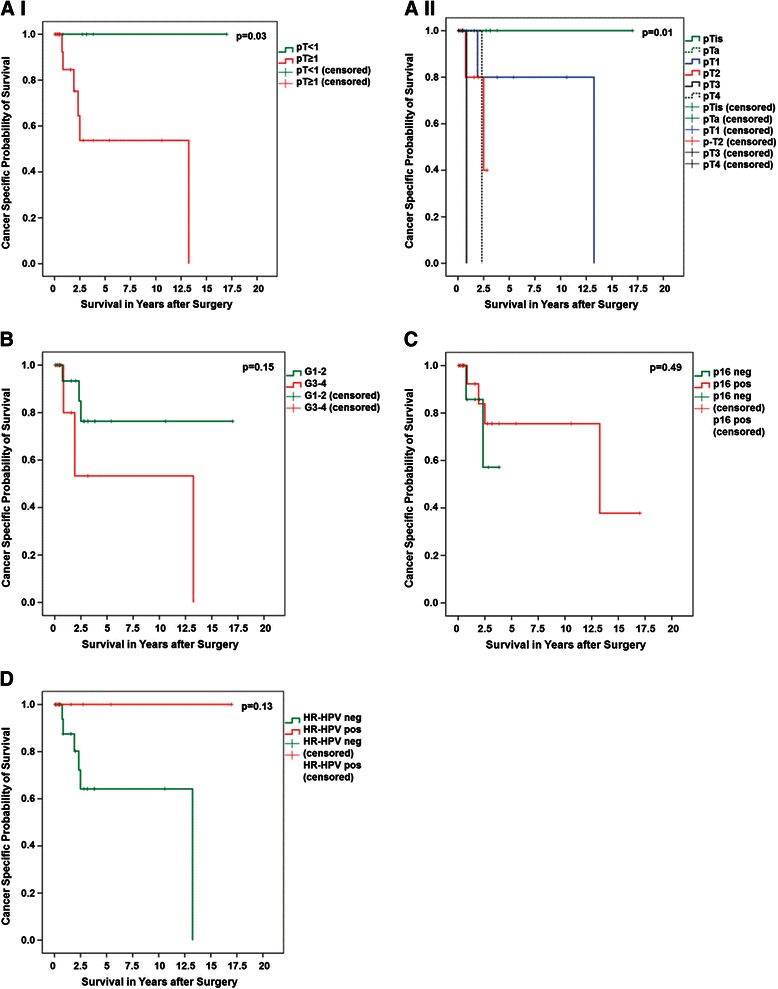
Cancer-specific survival analysis. A-I and -II, Cancer-specific survival depending on histopathologically confirmed T stage as indicated; B, depending on histopathologic differentiation grade; C, depending on p16INK4a expression; D, depending on HR-HPV status. G1-4, histopathologic differentiation grade; HR-HPV, high-risk human papillomavirus.
Discussion
The role of human high-risk papillomavirus infection as a causative agent in the development of penile squamous cell carcinoma is well established [20]. However, the impact of HPV-related tumourigenesis on tumour morphology as well as aggressiveness is still subject to research and has led to contradictory results [15-17]. Moreover, it is still not clear to which extent immunostaining for p16INK4a might be regarded as a surrogate marker for HPV infection, since site-specific differences in sensitivity and specificity have been reported [10,21-23]. The observed variety of staining patterns for p16INK4a further complicates histologic evaluation [24,25]. Therefore, the aim of this study was to evaluate p16INK4a staining patterns and results from HPV DNA subtyping with histopathological and clinical characteristics in a cohort of 58 patients with penile squamous cell carcinoma (pSCC). Among the study cohort, 91% of pSCCs were classified as conventional-type (keratinizing pSCC) because various degrees of keratinization could be observed in these cases. This is a higher proportion than has been described in earlier studies, while the rate of basaloid-type tumours was comparable to data from literature [11,16,26]. HPV DNA (of any subtype) was detected in 31% of all cases, with HR-HPV DNA (HPV-16 and HPV-45) being present in 28% of conventional-type and 40% of basaloid-type carcinomas. Thus, despite a specificity of 95% and a positive predictive value of 40%, the resulting sensitivity of basaloid tumour differentiation for the presence of HR-HPV DNA was only 11%. Due to the significant proportion of conventional-type (keratinizing) tumours that turned out HPV-positive in PCR-based assays, our results do not support the correlation between presence of HR-HPV DNA and basaloid tumour subtype that has been previously described by other authors. This might be due to the fact that distinguishing one histological type from another can be challenging, and overlapping differentiation patterns as well as intratumoural heterogeneity exist [27]. Therefore, we would not recommend to rely solely on basaloid tumour subtype when assessing hints for HPV-driven tumourigenesis as proposed by some authors but rather use a combination of criteria as proposed in a 2014 study by Chaux et al. [28,29]. Positive immunostaining for p16INK4a, a tumour suppressor protein that is regarded as a surrogate marker for HPV-associated tumours in other organs [7,30], was observed in 59% of specimens. There was a wide variety from scattered-focal over confluent-intense to almost exclusively nuclear staining patterns. P16INK4a immunostaining correlated significantly with the presence of HR-HPV DNA with good sensitivity (100%), but lacked specificity (60%) when all staining patterns were considered (PPV, 53%). Considering only those specimens with intense nuclear positivity for p16INK4a in all tumour cells improved the specificity for the presence of HR-HPV DNA to 85% (PPV, 75%). P16INK4a was present in all HPV-positive cases; however, lack of p16INK4a immunostaining in HPV-associated tumours due to loss of heterozygosity near the CDKN2A locus and/or hypermethylation of the CDKN2A promoter has been described previously [31]. It is therefore well conceivable that these genetic aberrations accumulate stepwise during tumour progression, and could not be observed here due to the relatively large proportion of early/noninvasive HPV-positive tumours in our study. Also, it has to be stated that we did not examine p16INK4a expression in corresponding metastases, but a 2014 study showed identical immunohistochemical or HPV in situ hybridization profiles between primary pSCCs and their corresponding metastases [32]. However, the question remains if there is a prognostic value of HR-HPV-driven tumourigenesis in pSCC at all. To address this, we investigated whether presence of HR-HPV DNA was associated with tumour aggressiveness or cancer-specific survival in pSCC. We found that HR-HPV as well as p16INK4a positivity was significantly associated with non-invasive tumour growth (pTis/pTa stage). This finding is in contrast to a proposed proinvasive role for HPV oncoproteins that has been recently described in head and neck as well as cervical cancer, but is supported by data for penile cancer published by other authors [13,16,33-35]. Our findings therefore add to the growing evidence that there are site-specific differences in the role of HPV regarding the gain of an invasive phenotype. These differences may be linked to interaction of HPV-derived oncoproteins with β-integrin localization and signalling [36]. For nodal or distant metastasis, there was no significant correlation with presence of HR-HPV DNA, p16INK4a staining or histologic grade in our sample set; similar results have been previously described [37]. Furthermore, we detected no significant differences in cancer-specific survival with regard to histologic differentiation grade, or p16INK4a positivity. This is in line with some previous studies that failed to confirm a proposed association between histopathologic grade and lymph node metastasis or overall survival in pSCC, while it has to be stated as a clear limitation of our study that follow-up data was only obtainable for 35 patients (60.3%) [38-40]. Considering the substantial interobserver variability that has been described for histopathologic grading in pSCC (between 59-87% with ĸ = 0.38-0.69 [41]), we think that the current histopathologic grading is of limited value due to an obvious lack of prognostic relevance; this conclusion is also supported by other authors [42]. For the presence of HR-HPV we observed a trend towards better prognosis that failed to reach statistical significance; further investigations in larger cohorts might therefor be indicated.
Conclusion
In this study, we evaluate histologic subtyping as well as p16INK4a immunostaining for detection of HR-HPV DNA and propose that ubiquitous nuclear expression of p16INK4a is a strong predictor for the presence of HR-HPV in penile squamous cell carcinoma. We further show that, in contrast to histologic grading, presence of HR-HPV DNA is associated with noninvasive growth of the tumour. However, since there was no significant impact of HPV-driven tumourigenesis on metastasis and cancer-specific survival in pSCC, routine p16INK4a immunostaining or HPV genotyping in pSCC cannot be recommended.
Acknowledgements
The authors would like to thank Claudia Schlosser and Anette Daniel for excellent technical assistance. No current external funding was obtained for this study.
Abbreviations
- HNSCCs
Squamous cell carcinomas of the head and neck region
- HR/LR-HPV
High-risk/ low-risk human papillomavirus
- PCR
Polymerase chain reaction
- PPV
Positive predictive value
- pSCC
penile squamous cell carcinoma
- UICC
Union internationale contre le cancer
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JS, AAG, TJS and KS identified appropriate tissue specimens, planned and supervised automated immunohistochemical staining and gathered clinical, pathological and molecular data. JS drafted the manuscript. JS, KS and PM reviewed H/E-stained as well as immunostained tissue slides. AA carried out DNA isolation and HPV subtyping. JS, AAG, MS, AJS and KS participated in the design of the study, and JS, AJS and KS performed the statistical analyses. JS, AAG, AJS and KS conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Julie Steinestel, Email: julie@steinestel.com.
Andreas Al Ghazal, Email: andreas.alghazal@uniklinik-ulm.de.
Annette Arndt, Email: annettearndt@bundeswehr.org.
Thomas J Schnoeller, Email: thomas.schnoeller@uniklinik-ulm.de.
Andres J Schrader, Email: ajschrader@gmx.de.
Peter Moeller, Email: peter.moeller@uniklinik-ulm.de.
Konrad Steinestel, Email: konrad@steinestel.com.
References
- 1.Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39(4):527–47. doi: 10.1111/dsu.12029. [DOI] [PubMed] [Google Scholar]
- 2.Micali G, Nasca MR, Innocenzi D, Schwartz RA. Penile cancer. J Am Acad Dermatol. 2006;54(3):369–91. doi: 10.1016/j.jaad.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116(4):606–16. doi: 10.1002/ijc.21009. [DOI] [PubMed] [Google Scholar]
- 4.Hartwig S, Syrjanen S, Dominiak-Felden G, Brotons M, Castellsague X. Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: a review. BMC Cancer. 2012;12:30. doi: 10.1186/1471-2407-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marty R, Roze S, Bresse X, Largeron N, Smith-Palmer J. Estimating the clinical benefits of vaccinating boys and girls against HPV-related diseases in Europe. BMC Cancer. 2013;13:10. doi: 10.1186/1471-2407-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110(5):525–41. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 7.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branca M, Ciotti M, Santini D, Di Bonito L, Giorgi C, Benedetto A, et al. p16(INK4A) expression is related to grade of cin and high-risk human papillomavirus but does not predict virus clearance after conization or disease outcome. Int J Gynecol Pathol. 2004;23(4):354–65. doi: 10.1097/01.pgp.0000139639.79105.40. [DOI] [PubMed] [Google Scholar]
- 9.Jordan RC, Lingen MW, Perez-Ordonez B, He X, Pickard R, Koluder M, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36(7):945–54. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinestel J, Cronauer MV, Muller J, Al Ghazal A, Skowronek P, Arndt A, et al. Overexpression of p16(INK4a) in urothelial carcinoma in situ is a marker for MAPK-mediated epithelial-mesenchymal transition but is not related to human papillomavirus infection. PLoS One. 2013;8(5):e65189. doi: 10.1371/journal.pone.0065189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaux A, Pfannl R, Lloveras B, Alejo M, Clavero O, Lezcano C, et al. Distinctive association of p16INK4a overexpression with penile intraepithelial neoplasia depicting warty and/or basaloid features: a study of 141 cases evaluating a new nomenclature. Am J Surg Pathol. 2010;34(3):385–92. doi: 10.1097/PAS.0b013e3181cdad23. [DOI] [PubMed] [Google Scholar]
- 12.O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48(12):1191–201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Au Yeung CL, Tsang TY, Yau PL, Kwok TT. Human papillomavirus type 16 E6 induces cervical cancer cell migration through the p53/microRNA-23b/urokinase-type plasminogen activator pathway. Oncogene. 2011;30(21):2401–10. doi: 10.1038/onc.2010.613. [DOI] [PubMed] [Google Scholar]
- 14.Jung YS, Kato I, Kim HR. A novel function of HPV16-E6/E7 in epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2013;435(3):339–44. doi: 10.1016/j.bbrc.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 15.Chaux A, Cubilla AL. The role of human papillomavirus infection in the pathogenesis of penile squamous cell carcinomas. Semin Diagn Pathol. 2012;29(2):67–71. doi: 10.1053/j.semdp.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Ferrandiz-Pulido C, Masferrer E, de Torres I, Lloveras B, Hernandez-Losa J, Mojal S, et al. Identification and genotyping of human papillomavirus in a Spanish cohort of penile squamous cell carcinomas: correlation with pathologic subtypes, p16(INK4a) expression, and prognosis. J Am Acad Dermatol. 2013;68(1):73–82. doi: 10.1016/j.jaad.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Scheiner MA, Campos MM, Ornellas AA, Chin EW, Ornellas MH, Andrada-Serpa MJ. Human papillomavirus and penile cancers in Rio de Janeiro, Brazil: HPV typing and clinical features. Int Braz J Urol. 2008;34(4):467–74. doi: 10.1590/S1677-55382008000400009. [DOI] [PubMed] [Google Scholar]
- 18.Thuret R, Sun M, Abdollah F, Budaus L, Lughezzani G, Liberman D, et al. Tumor grade improves the prognostic ability of American Joint Committee on Cancer stage in patients with penile carcinoma. J Urol. 2011;185(2):501–7. doi: 10.1016/j.juro.2010.09.111. [DOI] [PubMed] [Google Scholar]
- 19.Thomas J, Primeaux T. Is p16 immunohistochemistry a more cost-effective method for identification of human papilloma virus-associated head and neck squamous cell carcinoma? Ann Diagn Pathol. 2012;16(2):91–9. doi: 10.1016/j.anndiagpath.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Chaux A, Cubilla AL. Advances in the pathology of penile carcinomas. Hum Pathol. 2012;43(6):771–89. doi: 10.1016/j.humpath.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Wendt M, Romanitan M, Nasman A, Dalianis T, Hammarstedt L, Marklund L et al. Presence of human papillomaviruses and p16 expression in hypopharyngeal cancer. Head Neck. 2013. doi:10.1002/hed.23394. [DOI] [PubMed]
- 22.Alexander RE, Davidson DD, Lopez-Beltran A, Montironi R, MacLennan GT, Comperat E, et al. Human papillomavirus is not an etiologic agent of urothelial inverted papillomas. Am J Surg Pathol. 2013;37(8):1223–8. doi: 10.1097/PAS.0b013e3182863fc1. [DOI] [PubMed] [Google Scholar]
- 23.Cubilla AL, Lloveras B, Alejo M, Clavero O, Chaux A, Kasamatsu E, et al. Value of p16(INK)(4)(A) in the pathology of invasive penile squamous cell carcinomas: a report of 202 cases. Am J Surg Pathol. 2011;35(2):253–61. doi: 10.1097/PAS.0b013e318203cdba. [DOI] [PubMed] [Google Scholar]
- 24.Sawicka M, Pawlikowski J, Wilson S, Ferdinando D, Wu H, Adams PD, et al. The specificity and patterns of staining in human cells and tissues of p16INK4a antibodies demonstrate variant antigen binding. PLoS One. 2013;8(1):e53313. doi: 10.1371/journal.pone.0053313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen ZW, Weinreb I, Kamel-Reid S, Perez-Ordonez B. Equivocal p16 immunostaining in squamous cell carcinoma of the head and neck: staining patterns are suggestive of HPV status. Head Neck Pathol. 2012;6(4):422–9. doi: 10.1007/s12105-012-0382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaux A, Velazquez EF, Amin A, Soskin A, Pfannl R, Rodriguez IM, et al. Distribution and characterization of subtypes of penile intraepithelial neoplasia and their association with invasive carcinomas: a pathological study of 139 lesions in 121 patients. Hum Pathol. 2012;43(7):1020–7. doi: 10.1016/j.humpath.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Chaux A, Cubilla AL. Diagnostic problems in precancerous lesions and invasive carcinomas of the penis. Seminars in diagnostic pathology. Philadelphia: WB Saunders; 2012. pp. 72–82. [DOI] [PubMed] [Google Scholar]
- 28.Miralles-Guri C, Bruni L, Cubilla AL, Castellsague X, Bosch FX, de Sanjose S. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol. 2009;62(10):870–8. doi: 10.1136/jcp.2008.063149. [DOI] [PubMed] [Google Scholar]
- 29.Chaux A, Cubilla AL, Haffner MC, Lecksell KL, Sharma R, Burnett AL, et al. Combining routine morphology, p16INK4a immunohistochemistry, and in situ hybridization for the detection of human papillomavirus infection in penile carcinomas: A tissue microarray study using classifier performance analyses. Urol Oncol. 2014;32(2):171–7. doi: 10.1016/j.urolonc.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Tjalma WA, Fiander A, Reich O, Powell N, Nowakowski AM, Kirschner B, et al. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int J Cancer. 2013;132(4):854–67. doi: 10.1002/ijc.27713. [DOI] [PubMed] [Google Scholar]
- 31.Poetsch M, Hemmerich M, Kakies C, Kleist B, Wolf E, Vom Dorp F, et al. Alterations in the tumor suppressor gene p16 INK4A are associated with aggressive behavior of penile carcinomas. Virchows Arch. 2011;458(2):221–9. doi: 10.1007/s00428-010-1007-4. [DOI] [PubMed] [Google Scholar]
- 32.Mentrikoski MJ, Stelow EB, Culp S, Frierson HF, Jr, Cathro HP. Histologic and Immunohistochemical Assessment of Penile Carcinomas in a North American Population. Am J Surg Pathol. 2014;38(10):1340–8. doi: 10.1097/PAS.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 33.Kaewprag J, Umnajvijit W, Ngamkham J, Ponglikitmongkol M. HPV16 oncoproteins promote cervical cancer invasiveness by upregulating specific matrix metalloproteinases. PLoS One. 2013;8(8):e71611. doi: 10.1371/journal.pone.0071611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bezerra AL, Lopes A, Santiago GH, Ribeiro KC, Latorre MR, Villa LL. Human papillomavirus as a prognostic factor in carcinoma of the penis: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. Cancer. 2001;91(12):2315–21. doi: 10.1002/1097-0142(20010615)91:12<2315::AID-CNCR1263>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 35.Joo YH, Jung CK, Sun DI, Park JO, Cho KJ, Kim MS. High-risk human papillomavirus and cervical lymph node metastasis in patients with oropharyngeal cancer. Head Neck. 2012;34(1):10–4. doi: 10.1002/hed.21697. [DOI] [PubMed] [Google Scholar]
- 36.Holloway A, Storey A. A conserved C-terminal sequence of high-risk cutaneous beta-HPV E6 proteins alters localisation and signalling of beta1-integrin to promote cell migration. The Journal of general virology. 2013. doi:10.1099/vir.0.057695-0. [DOI] [PubMed]
- 37.da Fonseca AG, Soares FA, Burbano RR, Silvestre RV, Pinto LO. Human Papilloma Virus: Prevalence, distribution and predictive value to lymphatic metastasis in penile carcinoma. Int Braz J Urol. 2013;39(4):542–50. doi: 10.1590/S1677-5538.IBJU.2013.04.12. [DOI] [PubMed] [Google Scholar]
- 38.Liu JY, Li YH, Zhang ZL, Yao K, Ye YL, Xie D et al. The risk factors for the presence of pelvic lymph node metastasis in penile squamous cell carcinoma patients with inguinal lymph node dissection. World journal of urology. 2013. doi:10.1007/s00345-013-1024-4. [DOI] [PubMed]
- 39.Mannweiler S, Sygulla S, Tsybrovskyy O, Razmara Y, Pummer K, Regauer S. Clear-cell differentiation and lymphatic invasion, but not the revised TNM classification, predict lymph node metastases in pT1 penile cancer: a clinicopathologic study of 76 patients from a low incidence area. Urol Oncol. 2013;31(7):1378–85. doi: 10.1016/j.urolonc.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Al Ghazal A, Steffens S, Steinestel J, Lehmann R, Schnoeller TJ, Schulte-Hostede A, et al. Elevated C-reactive protein values predict nodal metastasis in patients with penile cancer. BMC Urol. 2013;13(1):53. doi: 10.1186/1471-2490-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunia S, Burger M, Hakenberg OW, May D, Koch S, Jain A, et al. Inherent grading characteristics of individual pathologists contribute to clinically and prognostically relevant interobserver discordance concerning Broders’ grading of penile squamous cell carcinomas. Urol Int. 2013;90(2):207–13. doi: 10.1159/000342639. [DOI] [PubMed] [Google Scholar]
- 42.Kakies C, Lopez-Beltran A, Comperat E, Erbersdobler A, Grobholz R, Hakenberg OW, et al. Reproducibility of histopathologic tumor grading in penile cancer—results of a European project. Virchows Arch. 2014;464(4):453–61. doi: 10.1007/s00428-014-1548-z. [DOI] [PubMed] [Google Scholar]


