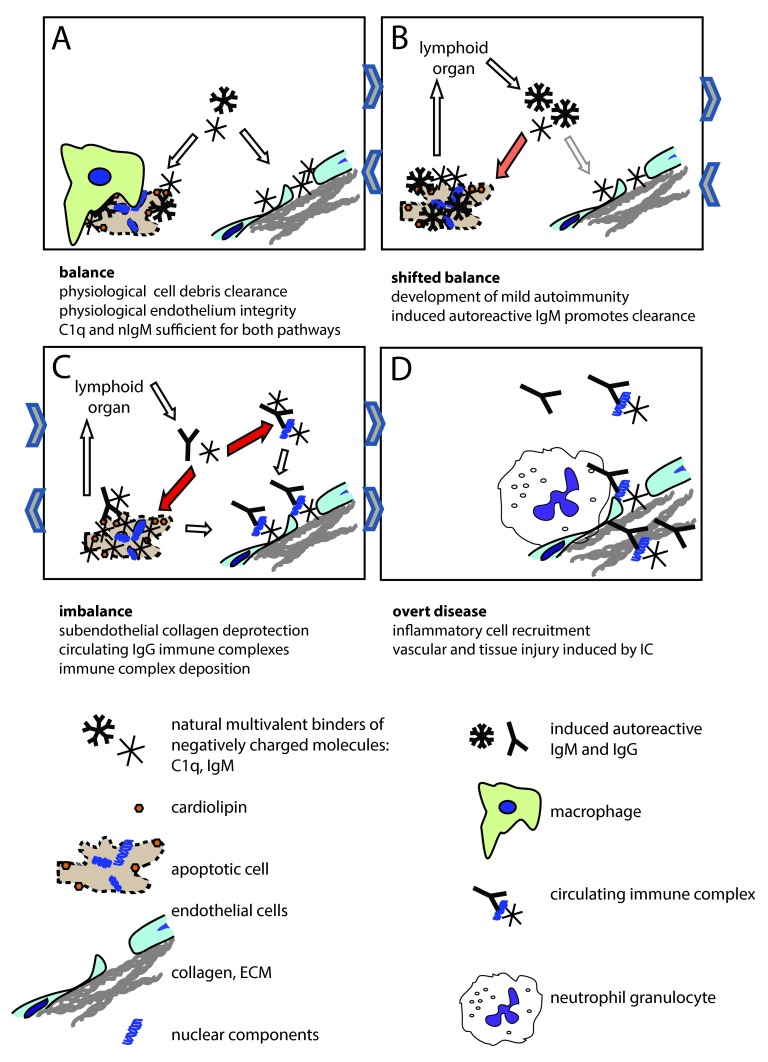
Figure 3. The endothelial deprotection hypothesis.
Maintenance of endothelial integrity and clearance of cellular debris both requires multimeric innate molecules (C1q, nIgM) with the ability to bind anionic surfaces ( A). C1q binds to fenestrated regions of the endothelial cell and to collagen exposed due to leaky endothelial junctions. Increased use of these innate molecules by the clearance mechanism, or deficiency of these molecules shifts this balance, which results in cellular debris reaching the lymphoid organs ( B). This triggers the production of IgM against the autoantigens. Induced autoreactive IgM binds to apoptotic autoantigens, activates the complement system and promotes clearance. Sustained autoantigenic stimulus and genetically determined clearance deficiency coupled with tendency of mounting inflammatory immune responses will result in the production of IgG against the tissue derived autoantigens ( C). Immune complexes containing IgG and C1q will bind to the vascular wall, since free C1q levels are low and circulating immune complexes will outcompete them. Deposited immune complexes containing IgG recruit white blood cells with Fcγ receptors, which can trigger cell activation, release of inflammatory cytokines, frustrated phagocytosis, NETosis ( D). Immune complexes and autoantibodies can penetrate the tissues via the damaged endothelium, causing organ specific damage. Sustained inflammation leads to irreversible organ damage. Orange and red arrows indicate mild and strong redirection of C1q usage, respectively. Arrows in between figure boxes indicate reversibility ( A– C) or irreversibility ( C– D).