Abstract
Purpose
Dendritic cell-associated C-type lectin-1 (Dectin-1) and dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) play a crucial role in the early procedure of fungal pathogen defenses. The present study evaluated the associations between Dectin-1 and DC-SIGN gene single nucleotide polymorphisms (SNPs) and susceptibility to fungal keratitis (FK) in the northern Han Chinese population.
Methods
The polymorphisms of Dectin-1 (rs17206002, rs3901533, rs11053613, and rs3901532) and DC-SIGN (rs4804803, rs2287886, rs735239, and rs735240) for 109 FK patients and 220 matched healthy controls were determined by PCR and DNA direct sequencing assay.
Results
Each SNP was consistent with Hardy–Weinberg equilibrium (p>0.05). The frequencies of genotypes and alleles for rs735239 and rs735240 (DC-SIGN) showed statistical differences between patients and control groups (p<0.05). The wild G allele of rs735239 and the wild A allele of rs735240 were significantly higher in patients (p = 0.003, OR = 1.766, 95% confidence interval [CI] 1.207–2.585; p = 0.014, OR = 1.609, 95% CI 1.100–2.355, respectively). No association with a risk of FK was found for the remaining SNPs (p>0.05) even after ruling out clinical characteristics, such as severity degree and case history. Carriers of the haplotype TC (rs4804803 and rs2287886) had a higher risk of developing fungal keratitis (p = 0.007, OR = 1.710, 95% CI 1.154–2.534). The distribution of haplotypes AG and GA (rs735239 and rs735240) between the two groups also showed significant differences (p = 0.014, p = 0.003, respectively).
Conclusions
Two SNPs of DC-SIGN (rs735239 and rs735240) are associated with susceptibility to FK in the northern Han Chinese population. The haplotypes of DC-SIGN may be susceptible to the risk of FK, whereas the analysis of Dectin-1 gene polymorphisms showed no significant association with FK risk. Further research with a larger sample is recommended.
Introduction
Fungal keratitis (FK) is recognized as one of most serious vision-threatening corneal infectious diseases because it can result in endophthalmitis, corneal perforation, and vision loss. Although new therapies, such as collagen cross-linking [1], voriconazole [2], and therapeutic keratoplasty [3], have emerged in clinical practice, their efficacies are still below expectations. Because of the penetrating ability of the fungal hyphae, pathogens can spread widely and deeply in the early stages, and graft re-infection may occur after corneal transplantation [4-6]. FK remains a therapeutic challenge to ophthalmologists because of the lack of established gold standard treatments and guidelines and because the incidence and etiology of FK are different among particular geographic areas. Due to many factors, such as the wide use of broad-spectrum antibiotics, immunosuppressants, and contact lens, FK has been reported more frequently in recent years [4,5], with Fusarium, Aspergillus, and Candida albicans being the main pathogens [4-9]. Therefore, effective preventive targets are necessary.
Innate immunity recognizes pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs), which could mediate the inflammatory response and play a pivotal role in anti-infection defense [10-12]. Early recognition of Aspergillus fumigatus is crucial for down-stream immune response and conidia clearance [12-14]. C-type lectin receptors (CLRs) are members of PRRs and are important in the response to fungal infection. CLRs, such as Dectin-1 and DC-SIGN, are considered as pivotal cell membrane PRRs in the innate immune system against fungal infection [14-16]. Studies have shown that nonimmune cells, such as human epidermal cells and corneal epithelium cells, can be involved in the antifungal immune response via inherent PRRs [10,11,13,17,18].
Genetic polymorphisms are recognized as potential factors that could affect human susceptibility and induce some infectious diseases, and some infectious diseases are correlated with environmental factors and gene polymorphisms [19,20]. Through genetic polymorphism studies, researchers have revealed the essence of individual differences in biologic function. Several studies have confirmed that Dectin-1 and DC-SIGN gene variants could result in altered cytokine release in fungal infection response [18-21], but little work has been carried out on the association between gene polymorphisms and FK. In this study, we investigated the distribution of Dectin-1 and DC-SIGN SNPs in the northern Han Chinese population and explored the relationships between these SNPs and susceptibility of FK.
Methods
Study population and classification
The study group consisted of 109 FK patients recruited from the Ophthalmology Department of Qingdao University Affiliated Hospital in Qingdao of China, from January 2008 to December 2012 (men 75; mean age: 54.61 ± 10.72 years). The control group comprised 220 unrelated healthy individuals (118 males; mean age 54.61±11.67 years) matched on gender and age from this hospital in the same period.
The diagnosis of FK was based on laboratory examinations (fungal culture) and clinical manifestation [22]. Among these patients, 68 cases had a history of corneal trauma. Patients were divided into three subgroups according to clinical features: mild (13 cases), defined as the area of corneal ulcer is smaller than 3 mm2 or the depth is less than one-third of the corneal thickness, a partial corneal edema, without an anterior chamber abscess; moderate (29 cases), defined as the area of corneal ulcer is 3–6 mm2 or the depth is between one-third and two-thirds of the corneal thickness, larger corneal edema, anterior chamber abscess; and severe (67 cases), defined as the corneal ulcer area is more than 6 mm2 or the depth is larger than two-thirds of the corneal thickness, cloudy corneal edema, folded Descemet's membrane, exudation and empyema in anterior chamber, significant vision loss, or unsuccessful drug treatment.
All the subjects were northern Han Chinese residents and had no relationship with each other. People with significant illness, such as type 2 diabetes mellitus, heart disease, cancer, hypertension, and atherosclerosis, were excluded from the study. There was no statistical difference in age and gender between the two groups (p>0.05). Informed consent of all participates was obtained, and the protocol of this study was approved by the Institutional Ethics Committee of Qingdao University Affiliated Hospital. All DNA samples and data in this study were handled anonymously.
Selection of SNPs and genotyping
Peripheral venous blood (2 ml) was collected in EDTA tube from all subjects. Genomic DNA was extracted using the genomic DNA purification kit (DP319, Tiangen Biotech CO., Beijing, China) following the manufacturer’s instruction. All genomic DNA was stored at -80 °C freezer until analysis. All single nucleotide polymorphisms (SNPs) were selected based on previous literature and information, including the NCBI GenBank, dbSNP, and HapMap databases. The selected SNPs were in 5’ noncoding region of Dectin-1 and the promoter region of DC-SIGN, which may influence gene transcription. Primers were designed with Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA) and synthesized by Sangon Biology company (Shanghai, China). PCR (Table 1) was performed in the Eppendorf PRO PCR system (Hamburg, German), using a PCR amplification kit (code No. RR003A, TaKaRa, Japan). All SNPs were genotyped by ABI 3730 XL system (Marjorbio Engineering Limited Company, Shanghai, China). DNA sequences were read by Chromas 2.3 software (Technelysium Pty Ltd., Tewantin, Australia). About 5% of samples were randomly selected and retested for internal quality control.
Table 1. Primer sequences for each SNP and PCR conditions.
| Gene | Gene location | SNPs | Primer pairs | Product length | Anneling temperature (°C) | Extendding time(s) | |
|---|---|---|---|---|---|---|---|
| Dectin-1 |
Intron |
rs17206002 rs3901533 rs11053613 rs3901532 |
F |
TGACTACAATAATCAGGACACTACC |
777 bp |
64 |
47 |
| R |
TAGCCATTGTCTTCTCCTCCA |
||||||
| DC-SIGN |
Promoter |
rs4804803 rs2287886 |
F |
ATTATGATTCTGCCCCAACTC |
424 bp |
63 |
27 |
| R |
CAGCTTTTATTTCCCACCCT |
||||||
| DC-SIGN | Promoter | rs735239 rs735240 | F |
TAGATGGTGGGGCTGAGACT |
407 bp | 62 | 25 |
| R | GCATACAGAAACCCCGTTGT | ||||||
Statistics
Each SNP in the controls was tested for Hardy–Weinberg equilibrium (HWE) by Haploview 4.2 (Massachusetts Institute of Technology, Cambridge, MA), and a value of p<0.05 was considered a deviation from HWE. Statistical comparison was performed by independent Student t test or one-way analysis of variance. Pearson’s χ2 or Bonferroni-corrected multiple comparison tests were used to identify differences in allele and genotype frequencies of the various SNPs and haplotypes between the patients and healthy controls. The odds ratios (ORs), 95% confidence intervals (CIs), and two-tailed p values were calculated for all tests. Statistical analysis was performed by SPSS 17.0 software (SPSS Inc., Chicago, IL). The level of significance for all statistical tests was defined as p<0.05.
Results
HWE and case-control genotype analyses
All SNPs of the healthy controls met HWE (p>0.05); the minor allele frequencies of all SNPs in this group were over 5%. The genotypes and allele frequencies are shown in Table 2. Differences in the genotypes and allele distributions of SNPs rs735239 and rs735240 (DC-SIGN) between the two groups were statistically significant (p<0.05). A significant under-representation of the rs735239 AA homozygote was observed in patients when compared to healthy controls (p = 0.019), whereas the rs735240 AA homozygote was significantly higher among patients (p = 0.033). The wild G allele of rs735239 and the wild A allele of rs735240 were significantly higher in FK patients (p = 0.003, OR = 1.766, 95% CI = 1.207–2.585; p = 0.014, OR = 1.609, 95% CI = 1.100–2.355, respectively). Other SNPs did not differ between the two groups.
Table 2. Genotype and allele distribution of FK patients compared with controls.
| SNP | Genotype/Allele | Cases (n=109) N(%) | Controls (n=220) N(%) | P* | OR (95%CI) ** |
|---|---|---|---|---|---|
| rs17206002 |
AA |
87 (79.8) |
184 (83.6) |
0.392 |
0.774(0.430–1.394) |
| TA |
22 (20.2) |
36 (16.4) |
|||
| T |
22 (10.1) |
36(8.2) |
0.416 |
0.794 (0.455–1.386) |
|
| A |
196 (89.9) |
404 (91.8) |
|||
| rs11053613 |
AA |
82 (75.2) |
169 (76.8) |
0.750 |
0.917(0.536–1.566) |
| GA |
27 (24.8) |
51 (23.2) |
|||
| G |
27 (12.4) |
51 (11.6) |
0.767 |
0.927 (0.564–1.525) |
|
| A |
191(87.6) |
389 (88.4) |
|||
| rs3901532 |
AA |
2 (1.8) |
6 (2.7) |
0.882 |
|
| GA |
41 (37.6) |
81 (36.8) |
|||
| GG |
66 (60.6) |
133 (60.5) |
|||
| A |
45 (20.6) |
93 (21.1) |
0.883 |
0.977 (0.711–1.341) |
|
| G |
173 (79.4) |
347 (78.9) |
|||
| rs3901533 |
GG |
2 (1.8) |
5 (2.3) |
0.949 |
|
| GT |
42 (38.5) |
82 (37.3) |
|||
| TT |
65 (59.6) |
133 (60.5) |
|||
| G |
46 (21.1) |
92 (20.9) |
0.955 |
1.009 (0.737–1.382) |
|
| T |
172 (78.9) |
348 (79.1) |
|||
| rs4804803 |
CC |
1 (0.9) |
5 (2.3) |
0.407 |
|
| CT |
17 (15.6) |
44 (20.0) |
|||
| TT |
91 (83.5) |
171 (77.7) |
|||
| C |
19 (8.7) |
54 (12.3) |
0.171 |
0.710 (0.432–1.167) |
|
| T |
199 (91.3) |
386 (87.7) |
|||
| rs2287886 |
CC |
14 (12.8) |
23 (10.5) |
0.407 |
|
| TC |
46 (42.2) |
81 (36.8) |
|||
| TT |
49 (45.0) |
116 (52.7) |
|||
| C |
74 (33.9) |
127 (28.9) |
0.183 |
1.176 (0.929–1.489) |
|
| T |
144 (66.1) |
313 (71.1) |
|||
| rs735239 |
AA |
73 (67.5) |
176 (80.0) |
0.019 |
|
| GA |
30 (27.5) |
40 (18.2) |
|||
| GG |
6 (5.5) |
4(1.8) |
|||
| G |
42 (19.3) |
48 (10.9) |
0.003 |
1.766 (1.207–2.585) |
|
| A |
176 (80.7) |
392 (89.1) |
|||
| rs735240 | AA |
12 (11.0) |
9 (4.1) |
0.033 |
|
| AG |
36 (33.0) |
66 (30.0) |
|||
| GG |
61 (56.0) |
145 (65.9) |
|||
| A |
60 (27.5) |
84 (19.1) |
0.014 |
1.609 (1.100–2.355) |
|
| G | 158 (72.5) | 356 (80.9) |
*: χ2 test; **: OR=odds ratio, CI=confidence interval.
Associations with severity classification and corneal trauma history
We divided the subjects into three groups based on disease severity and compared the genotype frequencies with controls (Table 3). The allele and genotype distributions of all SNPs in Dectin-1 and DC-SIGN did not show any trend of association with the severity of keratitis (p>0.05).
Table 3. Allele and genotype distribution by severity of FK.
| SNP | Genotype /Allele | Cases (n=109) N (%) |
Controls (n=220) N(%) | χ2 | P | ||
|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||||
| rs17206002 |
AA |
8(61.5) |
25 (86.2) |
54 (80.6) |
184 (83.6) |
4.567 |
0.206 |
| TA |
5 (38.5) |
4 (13.8) |
13 (19.4) |
36 (16.4) |
|||
| rs11053613 |
AA |
11 (84.6) |
22 (75.9) |
49 (73.1) |
169 (76.8) |
0.904 |
0.824 |
| GA |
2 (15.4) |
7 (24.1) |
18 (26.9) |
51 (23.2) |
|||
| rs3901532 |
AA |
0 (0.0) |
0 (0.0) |
2 (3.0) |
6 (2.7) |
7.532 |
0.274 |
| GA |
9 (69.2) |
9 (31.0) |
23 (34.3) |
81 (36.8) |
|||
| GG |
4 (30.8) |
20 (69.0) |
42 (62.7) |
133 (60.5) |
|||
| rs3901533 |
GG |
0 (0.00) |
0 (0.00) |
2(3.0) |
5 (2.3) |
7.246 |
0. 299 |
| GT |
9 (69.2) |
9 (31.0) |
24 (35.9) |
82 (37.3) |
|||
| TT |
4 (30.8) |
20(69.0) |
41(61.1) |
133(60.5) |
|||
| rs4804803 |
CC |
0 (0.0) |
1 (3.4) |
0 (0.0) |
5 (2.3) |
4.408 |
0. 622 |
| CT |
1 (7.7) |
6 (20.7) |
10 (14.9) |
44 (20.0) |
|||
| TT |
12 (92.3) |
22 (75.9) |
57 (85.1) |
171 (77.7) |
|||
| rs2287886 | CC |
1 (7.7) |
5 (17.2) |
8 (11.9) |
23 (10.5) |
4.740 |
0. 578 |
| TC |
4 (30.8) |
14 (48.3) |
28 (41.8) |
81 (36.8) |
|||
| TT | 8 (61.5) | 10 (34.5) | 31 (46.3) | 116 (52.7) | |||
We divided the cases into two groups according to whether or not the individual had a history of corneal trauma and compared this with the healthy controls (Table 4). No significant association was observed in the SNPs within Dectin-1 and DC-SIGN (p>0.05).
Table 4. Comparison the corneal trauma history cases with normal controls.
| SNP | Genotype/Allele | Cases (n=109) N (%) |
Contorls (n=220) N (%) | χ2 | P | |
|---|---|---|---|---|---|---|
| Corneal Trauma History (n=68) | No Corneal Trauma History (n=41) | |||||
| rs17206002 |
AA |
55(80.9) |
32(78.0) |
184 (83.6) |
0.874 |
0.646 |
| TA |
13(19.1) |
9(22.0) |
36 (16.4) |
|||
| rs11053613 |
AA |
51(75.0) |
31(75.6) |
169 (76.8) |
0.107 |
0.948 |
| GA |
17(25.0) |
10(24.4) |
51 (23.2) |
|||
| rs3901532 |
AA |
1(1.5) |
1(2.4) |
6(2.7) |
0.429 |
0.980 |
| GA |
25(36.8) |
16(39.0) |
81(36.8) |
|||
| GG |
42(61.8) |
24(59.5) |
133(60.5) |
|||
| rs3901533 |
GG |
1(1.5) |
1(2.4) |
5(2.3) |
0.235 |
0. 994 |
| GT |
26(38.2) |
16(39.0) |
82(37.3) |
|||
| TT |
41(60.3) |
24(58.5) |
133(60.5) |
|||
| rs4804803 |
CC |
0(0.0) |
1(2.4) |
5(2.3) |
3.074 |
0. 546 |
| CT |
12(17.6) |
5(12.2) |
44(20.0) |
|||
| TT |
56(82.4) |
35(85.4) |
171(77.7) |
|||
| rs2287886 |
CC |
7(10.3) |
7(17.1) |
23 (10.5) |
4.137 |
0.388 |
| TC |
32(47.1) |
14(34.1) |
81(36.8) |
|||
| TT |
29(42.6) |
20(48.8) |
116(52.7) |
|||
| rs735239 |
AA |
48(70.6) |
25(61.0) |
176(80.0) |
9.446 |
0.051 |
| GA |
17(25.0) |
13(31.7) |
40(18.2) |
|||
| GG |
3(4.4) |
3(7.3) |
4(1.8) |
|||
| rs735240 | AA |
7(10.3) |
5(12.2) |
9(4.1) |
7.346 |
0.119 |
| AG |
24(35.3) |
12(29.3) |
66(30.0) |
|||
| GG | 37(54.4) | 24(58.5) | 145(65.9) | |||
Linkage disequilibrium and haplotype analyses
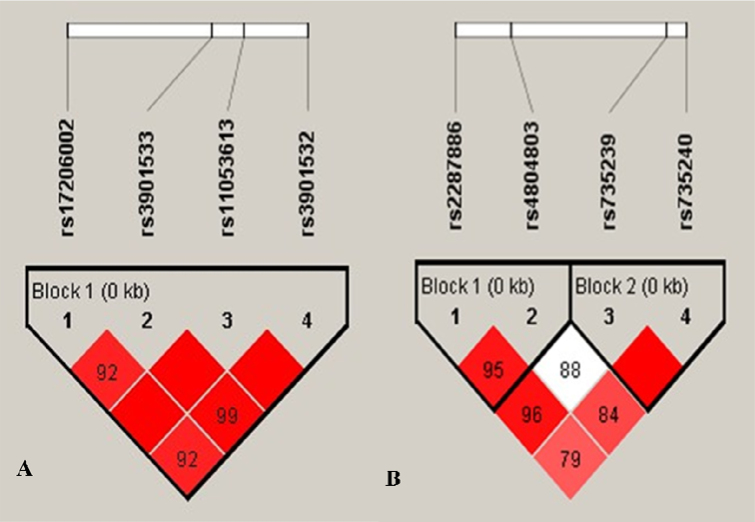
Linkage disequilibrium (LD) and haplotype analyses were performed with Haploview 4.2 to identify the genetic relationship between each SNP on Dectin-1 and DC-SIGN (Table 5 and Table 6). The haplotype analysis of the DC-SIGN gene region, which was divided into two LD blocks (block 1 included rs2287886 and rs4804803; block 2 included rs735239 and rs735240), revealed a strong association with FK and an extremely high magnitude of LD between the SNPs (pair-wise D' statistic >0.95) (Figure 1). Comparing the three haplotypes of DC-SIGN block 1, we found that in two groups Haplotype CT, which included a minor allele of rs2287886 and a major allele of rs4804803, was statistically significant (OR = 1.710, 95% CI = 1.154–2.534, p = 0.007), suggesting that Haplotype CT (rs2287886–rs4804803) was correlated with an increased risk of FK. Moreover, in block 2, Haplotypes AG and Haplotypes GA (rs735239–rs735240) showed significant differences between the cases and control subjects (p<0.05). Interestingly, Haplotypes AG, which included major alleles of rs735239 and rs735240, showed a reduced risk of FK, while Haplotypes GA, which included minor alleles of rs735239 and rs735240, showed an increased risk (Haplotypes AG: OR = 0.621, 95% CI = 0.425–0.909, p = 0.014; Haplotypes GA: OR = 1.949, 95% CI = 1.242–3.059, p = 0.003). However, as shown in Table 6, in the haplotype analysis of Dectin-1, one LD block (rs17206002, rs3901533, rs11053613, rs3901532) showed no significant association with FK (p>0.05).
Table 5. Haplotype analysis for DC-SIGN gene polymorphisms.
| No | Haplotype | Frequency |
OR (95% CI) | P | |||
|---|---|---|---|---|---|---|---|
| Total | Cases | Controls | |||||
| rs4804803 |
rs2287886 |
||||||
| H1 |
T |
T |
0.691 |
0.656 |
0.709 |
0.728 (0.553–1.107) |
0.165 |
| H2 |
T |
C |
0.198 |
0.257 |
0.168 |
1.710 (1.154–2.534) |
0.007 |
| H3 |
C |
C |
0.107 |
0.083 |
0.120 |
0.657 (0.375–1.152) |
0.140 |
| rs735239 |
rs735240 |
||||||
| H1 |
A |
G |
0.781 |
0. 725 |
0. 809 |
0.621 (0.425–0.909) |
0.014 |
| H2 |
G |
A |
0.137 |
0.193 |
0.109 |
1.949 (1.242–3.059) |
0.003 |
| H3 | A | A | 0.082 | 0.083 | 0.082 | 1.010 (0.560–1.823) | 0.974 |
Table 6. Haplotype analysis for Dectin-1 gene polymorphisms.
| No | Haplotype |
Frequency |
OR (95% CI) | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| rs17206002 | rs3901533 | rs11053613 | rs3901532 | Total | Cases | Controls | |||
| H1 |
A |
T |
A |
G |
0.670 |
0.665 |
0.673 |
0.966(0.685–1.364) |
0.845 |
| H2 |
A |
G |
A |
A |
0.123 |
0.110 |
0.130 |
0.831(0.501–1.380) |
0. 475 |
| H3 |
A |
T |
G |
G |
0.112 |
0.115 |
0.111 |
1.034(0.620–1.724) |
0.899 |
| H4 | T | G | A | A | 0.081 | 0.096 | 0.075 | 1.1315(0.741–2.332) | 0.348 |
Figure 1.
Linkage disequilibrium structure of the SNPs and haplotype blocks analyzed by Haploview 4.2 software. The D’ value of each SNP pair is expressed as a percentage and shown within the respective square (D’= 1 not shown). Higher D’ values are indicated in brighter red. A: LD plot of four SNPs of the DC-SIGN gene in 109 study subjects. The four SNPs constitute two haplotype blocks, with substantial LD among the SNPs of both blocks (block 1: D’ =0.95; block 2: D’=1.00). B: LD plot of four SNPs of the Dectin-1 gene in 109 study subjects. The four SNPs constitute a haplotype block spanning 0.2 kb of the Dectin-1 gene, and the magnitude of LD between each SNP was extremely high, with pair-wise D’ ≥0.92.
Discussion
This is the first study to investigate the association between Dectin-1 and DC-SIGN SNPs and FK in the northern Chinese Han population. The present study aimed to analyze the eight SNPs (rs17206002, rs3901533, rs11053613, rs3901532, rs4804803, rs2287886, rs735239 and rs735240) in FK patients and control subjects to identify relationships between them. Our results showed that two SNPs (rs735239 and rs735240) and three haplotypes (CT, AG, and GA) in DC-SIGN were significantly associated with FK, indicating that the DC-SIGN promoter polymorphisms contribute to the risk of FK.
The interaction between host PRRs and PAMPs of pathogenic microorganisms plays a key role in the innate immune response, which occurs at an early stage of fungal infection. PRRs can initiate the body's nonspecific immune response, recruit a large number of inflammatory cells, and trigger a specific immune response [12,14,23]. Dectin-1 and DC-SIGN are popular members of the CLR superfamily as Ca2+-dependent type II transmembrane protein receptors, which are closely related to antifungal innate immunity. Dectin-1 and DC-SIGN can recognize the β-1, 3/1, 6-glucans in the cell membrane of fungi and then bind with endogenous ligands on the surface of T cells and endothelial cells [24-26]. Many studies have shown that Dectin-1 and DC-SIGN are involved in the identification of fungal pathogens and in the induction of antifungal Th1 and Th17 immune responses [27-29].
In our study, we hypothesized that mutations in the Dectin-1 and DC-SIGN genes, which participate in immune reactions, are likely to lead to changes of corresponding encoded products and affect the host’s defense to fungal infection. It is well established that some elements in a gene’s noncoding region could affect gene transcription and translation [21,30-32]. The promoter region plays an important role in the regulation of gene transcription, and the regulation of promoters on transcription is determined by plural regulatory sequences. Although a single nucleotide substitution may not generate a new transcription recognition sequence, it may change the ability of the transcription factor binding with the corresponding loci.
As mentioned above, rs3901532 and rs3901533 are in the introns of the Dectin-1 gene. They are assumed to influence other neighboring loci to change the susceptibility of a fungal infection. However, we found no statistically significant difference in the relationship between Dectin-1 and FK. Studies have shown that some SNPs of Dectin-1 are associated with a host’s susceptivity to fungal infections, such as Aspergillus and Candida [19,32,33]. Because Dectin-1 mutations are rarely reported in the Chinese population, we suggest that our inconsistent result might stem from the differences between populations. Moreover, further study with a larger sample size is necessary. Dectin-1 activates immunologic effector cells via the c-rapidly accelerated fibrosarcoma proteins (Raf-1) or Myeloid differentiation factor 88 (MyD88)-dependent signaling pathways to produce a large number of cytokines and chemokines. Dectin-1 receptors showed protective effects in the mouse lung A. fumigatus infection model [29]. Brown et al. [34] found that Dectin-1 gene-deficient mice injected with Candida albicans recruited fewer inflammatory cells than wild-type mice, but the mortality of these mice with no Dectin-1 gene increased. Sainz J et al. [19] reported that Dectin-1 rs3901533T/T and rs7309123G/G genotype carriers had a significantly increased risk of invasive pulmonary Aspergillus infection (IPA) and a galactomannan sugar-positive ratio; however, the Dectin-1 mRNA expression level in rs7309123G/G genotype healthy carriers was significantly reduced, thus affecting the susceptibility of the IPA. Plantinga et al. studied the relationship between Dectin-1 Y238 X gene polymorphism and hematopoietic stem cell transplantation (HSCT) patients with Candida albicans colonization and found that patients who underwent HSCT because of malignant hematologic diseases often had invasive fungal infections with high morbidity and lethality. However, in sequential investigations of rheumatoid arthritis, they obtained different results [20,35]. These data confirm that Dectin-1 plays a crucial role in the antifungal immune response and that its gene polymorphisms can affect the susceptibility to fungal infection-related diseases.
rs735239, rs735240, rs4804803, and rs2287886 are located in the promoter region of DC-SIGN, and studies have found that DC-SIGN promoter polymorphisms are associated with certain infectious diseases [30,36]. We speculated that although these SNPs in the promoter region of DC-SIGN could not change the encoded amino acid sequence, they might affect promoter activity and change the expression efficiency of DC-SIGN, thereby affecting the host antifungal immune reactions and prognosis process. Our results show that DC-SIGN SNPs are related to FK as a risk factor. But the alleles and genotypes frequencies of rs4804803 and rs2287886 and TT/CC haplotypes have no significant correlation with this disease. We suggest, therefore, that Allele C is a mutated loci and a different LD in the promoter region may interfere with transcription and affect the DC-SIGN-mediated recognition of fungal pathogens. This is potentially important as it suggests that a therapy designed to counteract the functional susceptibility of DC-SIGN may be beneficial even when administered to mature individuals. The promoter region can protect the RNA from RNAase, improve the efficiency of transcription to mRNA, and affect the accuracy of translation. After ruling out factors such as age, gender, and disease severity, we found there was no association between SNPs and FK. Typical FK patients usually have an agricultural corneal trauma before the occurrence of fungal infection [4,5,37,38]; however, in our study, some patients who were without history of corneal trauma were diagnosed with FK. Our results demonstrate that there is no genetic difference between patients with and without a corneal trauma history and suggest that other risk factors for FK exist. This is an interesting puzzle to be explored in future studies. In addition, further experimental research is necessary to define the direct functional association between DC-SIGN polymorphism and the occurrence of FK.
Researchers have found that some DC-SIGN SNPs have correlations with Aspergillus, Mycobacterium tuberculosis, dengue virus, and hepatitis B virus infections. Juan Sainz et al. found that DC-SIGN rs2287886 A allele carriers showed a depressed trend of invasive pulmonary Aspergillus infection [19]. A study on tick-borne encephalitis patients in Russia reported that among patients who had central nervous system diseases, the frequencies of the rs2287886 AA homozygote and the A allele were increased compared with other groups [39]. In several studies on dengue virus infection in Asia, -336A /G (rs4804803) polymorphism in DC-SIGN (CD209) promoter has association with dengue disease and dengue hemorrhagic fever. The rs4804803 A/G genotype can affect the expression of DC-SIGN on the cell surface to enhance the immune response and reduce viral replication [40,41]. DC-SIGN SNPs are reported to be associated with a risk of Kawasaki disease in Taiwanese children, celiac disease in in the Spanish population, human immunodeficiency virus infection, tuberculosis, severe acute respiratory ayndromes, human cytomegalovirus infection and hepatitis C virus infection [42-48]. Among these findings, some results seem to differ as a result of race differences.
In summary, our data revealed that the SNPs of DC-SIGN influence the susceptibility of FK in the northern Han Chinese population. In interpreting our findings, the present study has certain methodological limitations, such as the small sample size and adoption of multiple test corrections. Since our subjects were all recruited from the northern Han Chinese population, the result might be affected by geographic, ethnic, and genetic background factors. However, the lack of a relationship between Dectin-1 and the pathogenesis of FK is not definitive from this study. Because there are several mutations in these genes, further replication studies on the genetic pathogenesis of FK in diverse ethnic groups are needed to confirm the importance of DC-SIGN and Dectin-1 polymorphisms in affecting the risk of FK. In conclusion, more replication studies with a larger sample size in different populations and further functional investigations are necessary to elucidate the mechanisms of DC-SIGN and Dectin-1 polymorphisms on the susceptibility of FK and to lead to novel pharmacological treatments for FK.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 81170825). We sincerely thank all of the participants for their participation in this study and all of the medical staff involved in sample collection and diagnosis in ophthalmic pathology laboratory of the affiliated hospital, Qingdao University. No authors have any commercial interest in the subject of the manuscript or in entities discussed in the manuscript. The principal author had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Qu, Che and Zhao. Acquisition of data: Qu, Wang, Du, Guo and Chen. Analysis and interpretation of data: Qu, Che, Gao and Wang. Drafting of the manuscript: Qu and Wang. Critical revision of the manuscript for important intellectual content: Che, Lin and Zhao. Statistical analysis: Qu, Wang and Liu. Obtained funding: Che and Zhao. Administrative, technical, and material support: Che and Zhao. Study supervision: Che and Zhao.
References
- 1.Li Z, Jhanji V, Tao X, Yu H, Chen W, Mu G. Riboflavin/ultravoilet light-mediated crosslinking for fungal keratitis. Br J Ophthalmol. 2013;97:669–71. doi: 10.1136/bjophthalmol-2012-302518. [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan T, Constantinou M, Jhanji V, Vajpayee RB. Factors affecting treatment outcomes with voriconazole in cases with fungal keratitis. Cornea. 2013;32:445–9. doi: 10.1097/ICO.0b013e318254a41b. [DOI] [PubMed] [Google Scholar]
- 3.Sharma N, Sachdev R, Jhanji V, Titiyal JS, Vajpayee RB. Therapeutic keratoplasty for microbial keratitis. Curr Opin Ophthalmol. 2010;21:293–300. doi: 10.1097/ICU.0b013e32833a8e23. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19:210–20. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Sun S, Jing Y, Han L, Zhang H, Yue J. Spectrum of fungal keratitis in central China. Clin Experiment Ophthalmol. 2009;37:763–71. doi: 10.1111/j.1442-9071.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Zhao G, Che C, Lin J, Li N, Jia W, Zhang Q, Jiang N, Hu L. Effect of corneal graft diameter on therapeutic penetrating keratoplasty for fungal keratitis. Int J Ophthalmol. 2012;5:698–703. doi: 10.3980/j.issn.2222-3959.2012.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 2010;6:e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Zhao G. Mechanism of the immune response to keratomycosis. Zhonghua Yan Ke Za Zhi. 2011;47:378–81. [PubMed] [Google Scholar]
- 9.Lu XH, Gao Y, Zhang L. DU M, Li SX, Wang T, Gao H. Aetiology analyses of 334 cases fungal keratitis. Zhonghua Yan Ke Za Zhi. 2013;49:12–5. [PubMed] [Google Scholar]
- 10.Che CY, Jia WY, Xu Q, Li N, Hu LT, Jiang N, Lin J, Wang Q, Zhao GQ. The roles of surfactant protein D during Aspergillus fumigatus infection in human corneal epithelial cells. Int J Ophthalmol. 2012;5:13–7. doi: 10.3980/j.issn.2222-3959.2012.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jie Z, Wu XY, Yu FS. Activation of Toll-like receptors 2 and 4 in Aspergillus fumigatus keratitis. Innate Immun. 2009;15:155–68. doi: 10.1177/1753425908101521. [DOI] [PubMed] [Google Scholar]
- 12.Luther K, Torosantucci A, Brakhage AA, Heesemann J, Ebel F. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell Microbiol. 2007;9:368–81. doi: 10.1111/j.1462-5822.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu ZJ, Zhao GQ, Wang Q, Che CY, Jiang N, Hu LT, Xu Q. Nucleotide oligomerization domain 2 contributes to the innate immune response in THCE cells stimulated by Aspergillus fumigates conidia. Int J Ophthalmol. 2012;5:409–14. doi: 10.3980/j.issn.2222-3959.2012.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano-Gómez D, Domínguez-Soto A, Ancochea J, Jimenez-Heffernan JA, Leal JA, Corbí AL. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin mediates binding and internalization of Aspergillus fumigatus conidia by dendritic cells and macrophages. J Immunol. 2004;173:5635–43. doi: 10.4049/jimmunol.173.9.5635. [DOI] [PubMed] [Google Scholar]
- 15.Reid DM, Gow NA, Brown GD. Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol. 2009;21:30–7. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors:shaping immune responses. Nat Rev Immunol. 2009;9:465–79. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Che CY, Li C, Gao A, Lin J, Zhang LL, Xu Q, Wang Q, Zhao GQ. Dectin-1 expression at early period of Aspergillus fumigatus infection in rat's corneal epithelium. Int J Ophthalmol. 2013;6:30–3. doi: 10.3980/j.issn.2222-3959.2013.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Koning HD, Rodijk-Olthuis D, van Vlijmen-Willems IM, Joosten LA, Netea MG, Schalkwijk J, Zeeuwen PL. A comprehensive analysis of pattern recognition receptors in normal and inflamed human epidermis: upregulation of dectin-1 in psoriasis. J Invest Dermatol. 2010;130:2611–20. doi: 10.1038/jid.2010.196. [DOI] [PubMed] [Google Scholar]
- 19.Sainz J, Lupiáñez CB, Segura-Catena J, Vazquez L, Ríos R, Oyonarte S, Hemminki K, Försti A, Jurado M. Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection. PLoS ONE. 2012;7:e32273. doi: 10.1371/journal.pone.0032273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T, Donnelly JP, Brown GD, Kullberg BJ, Blijlevens NM, Netea MG. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–32. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 21.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhary A, Singh K. Spectrum of fungal keratitis in North India. Cornea. 2005;24:8–15. doi: 10.1097/01.ico.0000126435.25751.20. [DOI] [PubMed] [Google Scholar]
- 23.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 24.Lee HM, Yuk JM, Shin DM, Jo EK. Dectin-1 is inducible and plays an essentialrole for mycobacteria-induced innate immune responses in airway epithelial cells. J Clin Immunol. 2009;29:795–805. doi: 10.1007/s10875-009-9319-3. [DOI] [PubMed] [Google Scholar]
- 25.Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R, 3rd, Kumamoto T, Edelbaum D, Morita A, Bergstresser PR, Takashima A. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–67. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- 26.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–85. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 27.Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, Rizzetto L, Mukaremera L, Preechasuth K, Cavalieri D, Kanneganti TD, van der Meer JW, Kullberg BJ, Joosten LA, Gow NA, Netea MG. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol. 2011;90:357. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–54. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 29.Zenaro E, Donini M, Dusi S. Induction of Th1/Th17 immune response by Mycobacterium tuberculosis: role of dectin-1, Mannose Receptor, and DC-SIGN. J Leukoc Biol. 2009;86:1393–401. doi: 10.1189/jlb.0409242. [DOI] [PubMed] [Google Scholar]
- 30.Boily-Larouche G, Milev MP, Zijenah LS, Labbé AC, Zannou DM, Humphrey JH, Ward BJ, Poudrier J, Mouland AJ, Cohen EA, Roger M. Naturally-occurring genetic variants in human DC-SIGN increase HIV-1 capture, cell-transfer and risk of mother-to-child transmission. PLoS ONE. 2012;7:e40706. doi: 10.1371/journal.pone.0040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Li C, Meng X, Zhu P, Tan D. Mutation analysis of DC-SIGN promoter in chronic hepatitis B patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:1052–8. doi: 10.3969/j.issn.1672-7347.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D'Angelo C, Pierini A, Pitzurra L, Falzetti F, Carotti A, Perruccio K, Latgé JP, Rodrigues F, Velardi A, Aversa F, Romani L, Carvalho A. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood. 2010;116:5394–402. doi: 10.1182/blood-2010-04-279307. [DOI] [PubMed] [Google Scholar]
- 33.Glocker EO, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschläger N, Gross O, Ruland J, Grimbacher B. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196:407–12. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plantinga TS, Fransen J, Takahashi N, Stienstra R, van Riel PL, van den Berg WB, Netea MG, Joosten LA. Functional consequences of DECTIN-1 early stop codon polymorphism Y238X in rheumatoid arthritis. Arthritis Res Ther. 2010;12:R26. doi: 10.1186/ar2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang K, Deng S, Lu W, Wang F, Jia S, Li F, Yu L, Chen M. Association between CD209 −336A/G and −871A/G polymorphisms and susceptibility of tuberculosis: a meta-analysis. PLoS ONE. 2012;7:e41519. doi: 10.1371/journal.pone.0041519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J Ophthalmol. 2003;51:315–21. [PubMed] [Google Scholar]
- 38.Singh G, Palanisamy M, Madhavan B, Rajaraman R, Narendran K, Kour A, Venkatapathy N. Multivariate analysis of childhood microbial keratitis in South India. Ann Acad Med Singapore. 2006;35:185–9. [PubMed] [Google Scholar]
- 39.Barkhash AV, Perelygin AA, Babenko VN, Brinton MA, Voevoda MI. Single nucleotide polymorphism in the promoter region of the CD209 gene is associated with human predisposition to severe forms of tick-borne encephalitis. Antiviral Res. 2012;93:64–8. doi: 10.1016/j.antiviral.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Chen RF, Liu JW, Lee IK, Lee CP, Kuo HC, Huang SK, Yang KD. DC-SIGN (CD209) Promoter −336 A/G polymorphism is associated with dengue hemorrhagic fever and correlated to DC-SIGN expression and immune augmentation. PLoS Negl Trop Dis. 2011;5:e934. doi: 10.1371/journal.pntd.0000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakuntabhai A, Turbpaiboon C, Casadémont I, Chuansumrit A, Lowhnoo T, Kajaste-Rudnitski A, Kalayanarooj SM, Tangnararatchakit K, Tangthawornchaikul N, Vasanawathana S, Chaiyaratana W, Yenchitsomanus PT, Suriyaphol P, Avirutnan P, Chokephaibulkit K, Matsuda F, Yoksan S, Jacob Y, Lathrop GM, Malasit P, Desprès P, Julier C.A variant in the CD209 promoter is associated with severity of dengue disease. Nat Genet 200537507–13.Epub 2005 Apr 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu HR, Chang WP, Wang L, Lin YJ, Liang CD, Yang KD, Kuo CM, Huang YC, Chang WC, Kuo HC. DC-SIGN (CD209) promoter −336 A/G (rs4804803) polymorphism associated with susceptibility of Kawasaki disease. ScientificWorldJournal. 2012;2012:634835. doi: 10.1100/2012/634835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Núñez C, Rueda B, Martínez A, Maluenda C, Polanco I, López-Nevot MA, Ortega E, Sierra E, Gómez de la Concha E, Urcelay E, Martín J. A functional variant in the CD209 promoter is associated with DQ2-negative celiac disease in the Spanish population. World J Gastroenterol. 2006;12:4397–400. doi: 10.3748/wjg.v12.i27.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalmasso C, Carpentier W, Meyer L, Rouzioux C, Goujard C, Chaix ML, Lambotte O, Avettand-Fenoel V, Le Clerc S, de Senneville LD, Deveau C, Boufassa F, Debré P, Delfraissy JF, Broet P, Theodorou I. ANRS Genome Wide Association 01. Distinct genetic loci control plasma HIV-RNA and cellular HIV-DNA levels in HIV-1 infection: the ANRS Genome Wide Association 01 study. PLoS ONE. 2008;3:e3907. doi: 10.1371/journal.pone.0003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan KY, Xu MS, Ching JC, Chan VS, Ip YC, Yam L, Chu CM, Lai ST, So KM, Wong TY, Chung PH, Tam P, Yip SP, Sham P, Lin CL, Leung GM, Peiris JS, Khoo US. Association of a single nucleotide polymorphism in the CD209 (DC-SIGN) promoter with SARS severity. Hong Kong Med J. 2010;16(Suppl 4):37–42. [PubMed] [Google Scholar]
- 46.Vannberg FO, Chapman SJ, Khor CC, Tosh K, Floyd S, Jackson-Sillah D, Crampin A, Sichali L, Bah B, Gustafson P, Aaby P, McAdam KP, Bah-Sow O, Lienhardt C, Sirugo G, Fine P, Hill AV. CD209 genetic polymorphism and tuberculosis disease. PLoS ONE. 2008;3:e1388. doi: 10.1371/journal.pone.0001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng R, Zhou Y, Qin L, Jin R, Wang J, Lu J, Wang W, Tang S, Hu Z. Relationship between polymorphism of DC-SIGN (CD209) gene and the susceptibility to pulmonary tuberculosis in an eastern Chinese population. Hum Immunol. 2011;72:183–6. doi: 10.1016/j.humimm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mezger M, Steffens M, Semmler C, Arlt EM, Zimmer M, Kristjanson GI, Wienker TF, Toliat MR, Kessler T, Einsele H, Loeffler J. Investigation of promoter variations in dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN) (CD209) and their relevance for human cytomegalovirus reactivation and disease after allogeneic stem-cell transplantation. Clin Microbiol Infect. 2008;14:228–34. doi: 10.1111/j.1469-0691.2007.01902.x. [DOI] [PubMed] [Google Scholar]