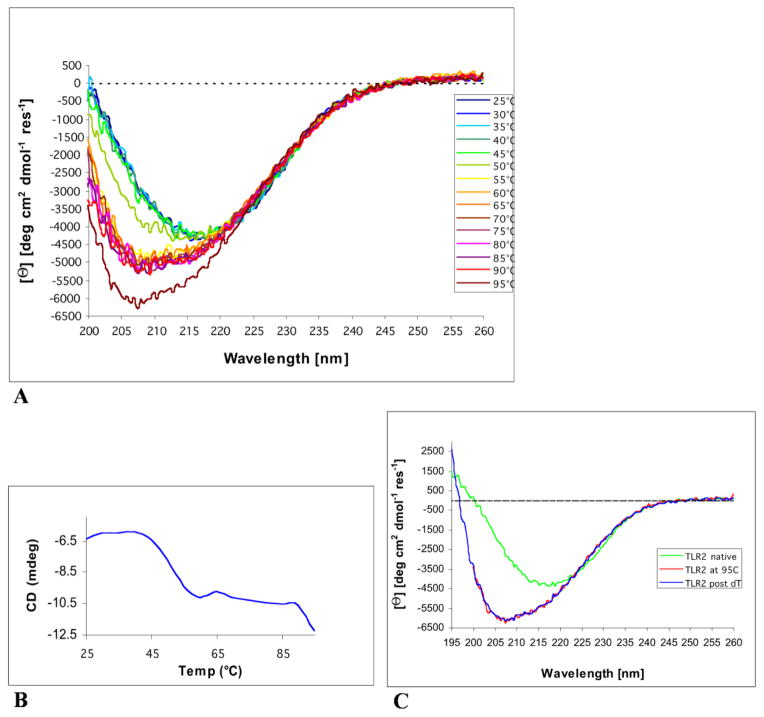
Figure 5. Circular Dichroism analysis of hTLR2ED.
A) CD spectra of hTLR2ED recorded at various temperatures. The CD spectrum of hTLR2ED at 25°C reveals a minimum around 215 nm that is indicative of a folded protein with a significant portion of β-sheet secondary structure. At temperatures greater than 50°C, the β-sheet character of the hTLR2ED CD spectra is lost and the shape of the spectra indicates that the hTLR2ED is now mostly unfolded. B) Thermal denaturation of hTLR2ED is a two-stage process. The CD signal at 208 nm was plotted against the corresponding temperatures. hTLR2ED undergoes two unfolding transitions: at the first Tm of 45–55°C, and a second Tm of 90–95°C, the protein becomes mostly unfolded. C) The thermal denaturation of hTLR2ED is irreversible. After the CD spectra of hTLR2ED were recorded at 95°C, hTLR2ED was slowly cooled back down to 25°C and new CD spectra were recorded (TLR2 post dT). There were no differences between the spectra at 95°C and after re-cooling, indicating that no secondary structure is regained and that the thermal denaturation of hTLR2ED is irreversible.