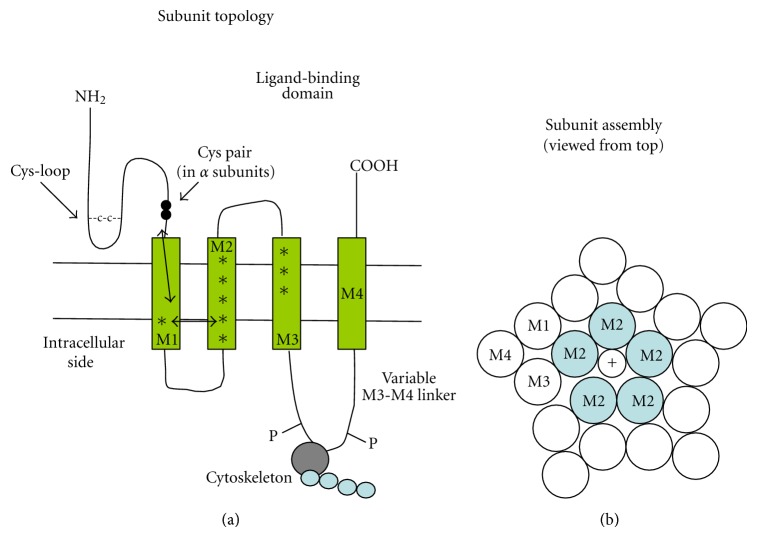
Figure 1.
Schematic of the location of ADNFLE mutations within the nAChR subunit structure. (a) overall topology of the typical nAChR subunit; asterisks mark the location of the mutations listed in Table 2. Double arrows mark the probable transduction pathway between the ligand-binding pocket and the M2 segment, which constitutes at the same time the channel gate and the selectivity filter. The panel also shows the location of the Cys-loop and the Cys pair that defines the α subunits. The M3-M4 variable linker is implicated in channel interaction with the cytoskeleton and regulation by phosphorylation. (b) probable arrangement of the M1–M4 segments of the five subunits constituting the pentameric receptor. The M2 segments line the channel pore. On agonist binding, the ligand pocket partially rotates. Such conformational change is transferred to the M2 segments, whose rotation removes from the channel lumen several hydrophobic amino acid side chains. In this way, the pore diameter widens from about 0.3 nm to approximately 0.8 nm. This enlargement is accompanied by the movement of hydrophilic groups into the lumen. The overall effect is considerable increase in ion permeability. For introduction to the structure-function studies on nAChRs, see [7, 8, 12, 14, 15].