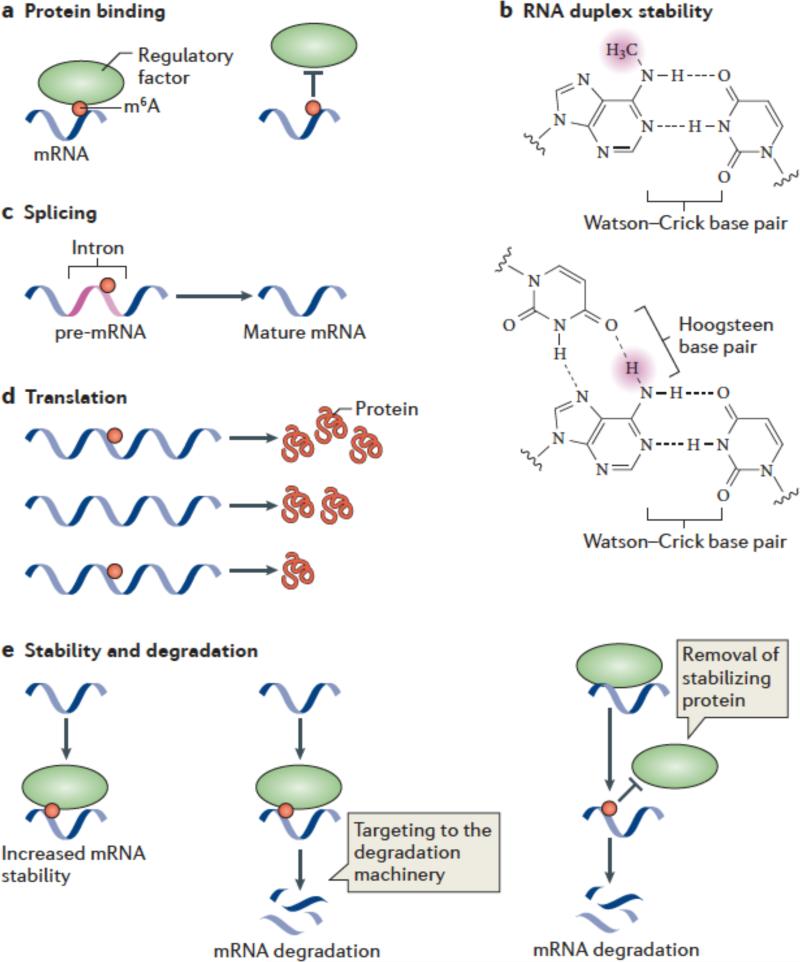
Figure 3. Mechanisms and functions of m6A.
(a,b) Several mechanisms have been ascribed to m6A. m6A is likely to have a role in facilitating or blocking RNA—protein interactions (a). m6A can also affect RNA by altering RNA structure or folding (b). In standard Watson-Crick base pairing, m6A is capable of pairing with uridine. Since a free proton at the N6 position is still available for hydrogen bonding, N6-methyladenosine behaves like adenosine in its ability to base pair to uridine. The squiggly line refers to the position of the ribose sugar to which the base is attached.
m6A interferes with the formation of base triples. As is shown in this U•A-U base triple, two free protons are required at the N6 position for Hoogsteen base pairing on one face and Watson-Crick base pairing on the other. The presence of a methyl group in place of one of these protons blocks base-triple formation. Thus, m6A can disrupt RNA structures dependent on base triples.
c-e) Great efforts are underway to determine the effects of m6A on mRNA fate and function. It has been suggested that m6A is targeted to specific intronic regions in order to influence splicing efficiency (c). Adenosine methylation may also lead to increased or reduced translation compared with unmethylated transcripts (d). m6A might promote mRNA degradation by recruiting proteins which target mRNAs toward the cellular degradation machinery. Alternatively, m6A might stabilize mRNAs by binding to proteins that promote transcript stability.