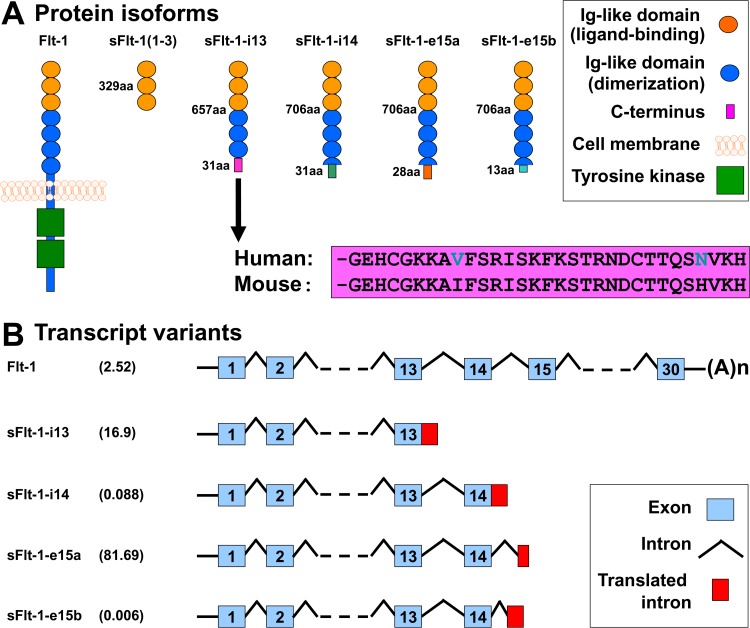
Fig 1. FLT1 protein isoforms and mRNA transcript variants.
(A) Flt-1 contains seven extracellular Ig-like domains and an intracellular tyrosine kinase. The first three extracellular Ig-like domains are essential for ligand-binding, while the 4–7th extracellular Ig-like domains for receptor dimerization. The truncated mouse sFlt-1 mutant [msFlt-1(1–3)] contains only 1–329 amino acids of Flt-1, corresponding to the first three Ig-like domains. Mouse and human sFlt-1-i13 contains the first six Ig-like domains corresponding to 1–657 amino acids of Flt-1, as well as a unique 31-amino-acid tail. This unique C-terminus is evolutionarily highly conserved among mammals; the mouse and human amino acid sequences of this tail are only different in two positions (shown with blue letters). Among the human placental expressed sFlt-1 isoforms, hsFlt-1-i14, hsFlt-1-e15a and hsFlt-1-e15b diverge from Flt-1 after amino acid 706, and contain a 31-, 28- and 13-amino-acid unique tails, respectively. (B) Among the placental expressed FLT1 transcripts, the abundance of the mRNA encoding for the transmembrane receptor is about 2.5% in preeclampsia. FLT1 transcript expression data was retrieved from Jebbink et al. and is shown as transcript level divided by total FLT1 transcript level [154]. HsFlt-1-i13, the second most abundant placental FLT1 transcript in preeclampsia, is generated by skipped splicing and extension of exon 13. Similarly, hsFlt-1-i14 is generated by skipped splicing and extension of exon 14. HsFlt-1-e15a and hsFlt-1-e15b contain alternatively spliced exons derived from intronic sequences (exon 15a and exon 15b, respectively). The most abundant placental transcript, hsFlt-1-e15a contains exon 15a, which is located within a primate-specific AluSeq retrotransposon. The graph was adapted with permission from figures in publications of Heydarian et al. [153] and Shibuya M. [243]. Permissions for reuse of these original figures were obtained from Elsevier Ltd. and from the Proceedings of the Japan Academy, Series B, respectively.