Figure 1.
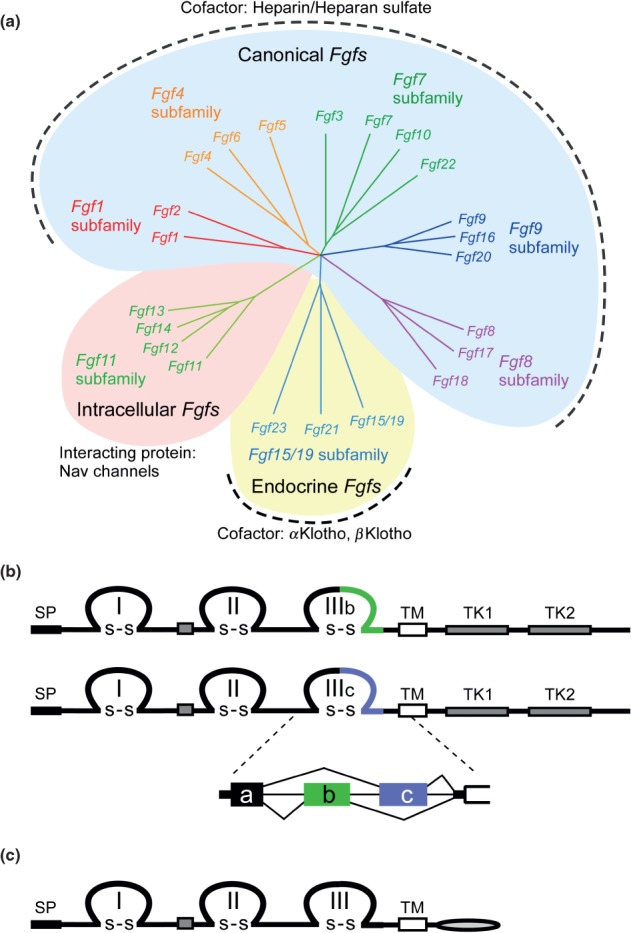
FGF and FGFR families. (a) Phylogenetic analysis suggests that 22 Fgf genes can be arranged into seven subfamilies containing two to four members each. Branch lengths are proportional to the evolutionary distance between each gene. The Fgf1, Fgf4, Fgf7, Fgf8, and Fgf9 subfamily genes encode secreted canonical FGFs, which bind to and activate FGFRs with heparin/HS as a cofactor. The Fgf15/19 subfamily members encode endocrine FGFs, which bind to and activate FGFRs with the Klotho family protein as a cofactor. The Fgf11 subfamily genes encode intracellular FGFs, which are non-signaling proteins serving as cofactors for voltage gated sodium channels and other molecules. (b) Schematic representations of FGFR protein structures are shown. FGFR is a receptor tyrosine kinase of ∼800 amino acids with several domains including three extracellular immunoglobulin-like domains (I, II, and III), a transmembrane domain (TM), and two intracellular tyrosine kinase domains (TK1 and TK2). SP indicates a cleavable secreted signal sequence. The Fgfr gene family is comprised of four members, Fgfr1-Fgfr4. Among them, Fgfr1–Fgfr3 generate two major splice variants of immunoglobulin-like domain III, referred to as IIIb and IIIc, which are essential determinants of ligand-binding specificity. (c) The schematic representation of FGFRL1/FGFR5 protein structure is shown. FGFRL1, with structural similarity to FGFRs, is a membrane protein of ∼500 amino acids with three extracellular immunoglobulin-like domains (I, II, and III), a transmembrane domain (TM), and a short intracellular tail with no tyrosine kinase domain. SP indicates a cleavable secreted signal sequence.
