Figure 3.
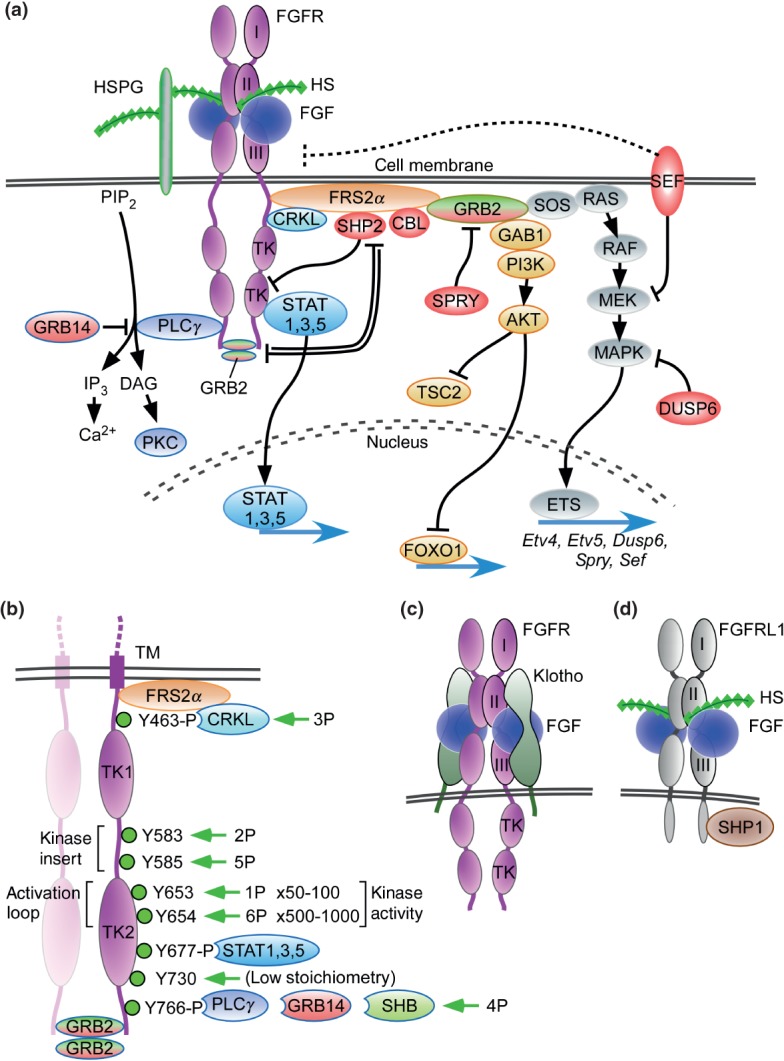
FGF signaling pathways. (a) Binding of canonical FGFs to FGFR with HS (or HSPG) as a cofactor induces the formation of ternary FGF-FGFR-HS complex, which activates the FGFR intracellular tyrosine kinase domain by phosphorylation of specific tyrosine residues. The activated receptor is coupled to intracellular signaling pathways including the RAS-MAPK, PI3K-AKT, PLCγ, and STAT pathways. The RAS-MAPK pathway: The major FGFR kinase substrate, FRS2α, which is constitutively associated with the juxtamembrane region of FGFR (peptide: MAVHKLAKSIPLRRQVTVSADS), interacts with CRKL bound to pY463 and is phosphorylated by the activated FGFR kinase. Phosphorylated FRS2α recruits the adaptor protein GRB2, which then recruits the guanine nucleotide exchange factor SOS. The recruited SOS activates the RAS GTPase, which then activates the MAPK pathway. MAPK activates members of the Ets transcription factor family such as Etv4 (Pea3) and Etv5 (Erm) and negative regulators of the FGF signaling pathways such as SHP2, CBL, SPRY, SEF, and DUSP6. The PI3-AKT pathway: The recruited GRB2 also recruits the adaptor protein GAB1, which then activates the enzyme PI3K, which then phosphorylates the enzyme AKT. AKT has multiple activities including activation of the mTOR complex 1 through inhibition of TSC2 and phosphorylation of the FOXO1 transcription factor causing it to exit the nucleus. The PLCγ pathway: Activated FGFR kinase recruits and activates the enzyme PLCγ, which produces IP3 and DAG by the hydrolysis of PIP2. IP3 induces calcium ion release from intracellular stores and the activation of downstream signaling pathways. DAG activates the enzyme PKC and its downstream signaling pathways. GRB14 inhibits activation of PLCγ. The STAT pathway: FGFR kinase also activates STAT1, 3, and 5. STAT3 interacts with phosphorylated tyrosine 677 (pYxxQ motif). These activated signaling pathways mostly regulate gene expression in the nucleus. SPRY interacts with GRB2 to inhibit the RAS-MAPK pathway and to regulate the PI3K-AKT pathway. GRB2 dimers are docked at the c-terminus of FGFR2 where they inhibit SHP2, allowing low-level receptor kinase activity. Molecules shaded red generally function to inhibit FGFR signaling. (b) Dimerization of the FGFR1 kinase domain leads to sequential phosphorylation of tyrosine residues (1P–6P) leading to increasing activity of the FGFR kinase and phosphorylation of tyrosine substrates for CRKL, STAT, GRB14, and PLCγ binding. In the first phase of activation, Y653 (1P), in the activation loop, is phosphorylated, resulting in a 50- to 100-fold increase in kinase activity. In the third phase of activation, Y654 (6P), in the activation loop, is phosphorylated, resulting in an overall 500–1000 fold increase in kinase activity. Y730 is weakly phosphorylated. Phosphorylation of Y677 allows docking of STAT3 and phosphorylation of Y766 allows docking of either GRB14 or PLCγ. Ligand-induced receptor activation phosphorylates GRB2, leading to its dissociation from the receptor. Tyrosine residues correspond to human FGFR1 (accession NP_075598). (c) Binding of endocrine FGF to FGFR with Klotho as a cofactor induces the formation of ternary FGF-FGFR-Klotho complex, which leads to activation of the FGFR tyrosine kinase. (d) FGFRL1 is a protein containing three extracellular immunoglobulin-like domains with similarity to FGFRs. FGFRL1 has a single transmembrane domain, and a short intracellular tail with no tyrosine kinase domain. The short cytoplasmic domain contains an SH2 binding motif that interacts with SHP1. FGFRL1 is not simply a decoy receptor, but rather a non-tyrosine kinase signaling molecule.
