Abstract
Introduction
The sympathetic activation is considered to be the main mechanism involved in the development of cardiovascular diseases in obstructive sleep apnea (OSA). The heart rate variability (HRV) analysis represents a non-invasive tool allowing the study of the autonomic nervous system. The impairment of HRV parameters in OSA has been documented. However, only a few studies tackled the dynamics of the autonomic nervous system during sleep in patients having OSA.
Aims
To analyze the HRV over sleep stages and across sleep periods in order to clarify the impact of OSA on cardiac autonomic modulation. The second objective is to examine the nocturnal HRV of OSA patients to find out which HRV parameter is the best to reflect the symptoms severity.
Methods
The study was retrospective. We have included 30 patients undergoing overnight polysomnography. Subjects were categorized into two groups according to apnea–hypopnea index (AHI): mild-to-moderate OSAS group (AHI: 5–30) and severe OSAS group (AHI>30). The HRV measures for participants with low apnea–hypopnea indices were compared to those of patients with high rates of apnea–hypopnea across the sleep period and sleep stages.
Results
HRV measures during sleep stages for the group with low rates of apnea–hypopnea have indicated a parasympathetic activation during non-rapid eye movement (NREM) sleep. However, no significant difference has been observed in the high AHI group except for the mean of RR intervals (mean RR). The parasympathetic activity tended to increase across the night but without a statistical difference. After control of age and body mass index, the most significant correlation found was for the mean RR (p=0.0001, r=−0.248).
Conclusion
OSA affects sympathovagal modulation during sleep, and this impact has been correlated to the severity of the disease. The mean RR seemed to be a better index allowing the sympathovagal balance appreciation during the night in OSA.
Keywords: autonomic nervous system, sleep apnea, heart rate, sleep, circadian
Obstructive sleep apnea (OSA) is a common disorder affecting at least 2–4% of the adult population (1, 2). It is characterized by the repetitive complete or partial collapses of the pharyngeal airway during sleep.
The diagnosis of OSA requires the presence of nocturnal and diurnal symptoms associated with an apnea–hypopnea index (AHI) greater than 5 events per hour on polysomnography (PSG). The presence of 15 or more obstructive respiratory events per hour of sleep in the absence of symptoms is also sufficient for the diagnostic (1).
It is known that the risk of cardiovascular diseases is increased in OSA (2, 3). The sympathetic activation is considered to be the main mechanism involved in the development of cardiovascular diseases (4, 5).
The heart rate variability (HRV) analysis represents a non-invasive tool allowing the study of the autonomic nervous system (6). HRV can be measured by different methods. Time-domain, frequency (spectral)-domain, and geometrical methods are the most common ones. In the time-domain method, heart rate taken at any time or distance between consequent normal complexes is determined. In the frequency-domain analysis, recordings are evaluated either in 2–5 min or in 24-h time intervals. Frequency-domain method is analyzed by studying the power spectral density. It provides information about the power distribution across frequencies (6). Three main spectral components are calculated.
The main two components are the low-frequency band (LF) and the high-frequency band (HF). Some investigators have accepted the LF to be an indicator for sympathetic activity. In contradiction, HF is determined by vagal effect and respiratory sinus arrhythmia. The LF/HF ratio has been derived from these values to show sympathovagal balance (6). The HRV can be used as a marker of autonomic modulation of the heart (7). A significant relationship between the HRV decrease and the cardiovascular risk has been demonstrated and reported in several diseases (8).
Several studies report a circadian rhythm of HRV. In fact, compared to wakefulness, the heart rate of a normal adult slows down during sleep (9, 10). However, during rapid-eye movement (REM) sleep, the heart rate does not differ significantly during the wake state, but during non-REM (NREM) sleep, the heart rate decreases progressively across the sleep periods. This pattern has been associated with progressive sympathetic inhibition during sleep, parasympathetic activation during NREM sleep, and parasympathetic inhibition during REM sleep (9). In patients presenting OSA, an imbalanced cardiac autonomic function has been documented (11–14).
Several studies have shown that the HRV analysis has the potential of distinguishing the OSA severity. The HRV has also been proposed as an alternative diagnostic tool (15, 16). However, few studies tackled the dynamics of the autonomic nervous system during sleep in patients having OSA (9).
The aim of this study is to analyze the HRV over sleep stages and across sleep periods in order to clarify the OSA impact on cardiac autonomic modulation. The second objective is to examine the nocturnal HRV of OSA patients to find out which parameter in time- or frequency-domain analysis is the best to reflect the severity of symptoms.
Methods
Study design
The study has been planned as a retrospective cohort study.
Study population
Data of 141 male patients admitted in our sleep laboratory for snoring and PSG performed between the period of January 2006 and March 2013 were analyzed.
The subjects met the following inclusion criteria: male, aged between 18 and 60 years old, the apnea–hypopnea index greater than 5 events per hour with clinical symptoms or only greater than 15 events per hour (1). The choice of including men only was an aim to guarantee the group homogeneity and due to the absence of information concerning the female hormonal status (17, 18). Patients known to have cardiovascular, renal, hepatic diseases, thyroid dysfunction, diabetes, a history of endocrinopathy or drug use that interact with the autonomic nervous system, a history of operations or CPAP treatment for OSA, periodic limb movements during sleep (PLMS), were excluded from the protocol.
Basing on these criteria, we have analyzed the records of 66 patients included in the protocol. At this stage, HRV analysis has been performed in 30 patients with their consent, excluding 36 cases having artifacts and ectopic heart beats in their ECG or having less than three sleep NREM–REM cycles. The subjects were categorized into two groups: mild-to-moderate OSA group (n=16) and severe OSA group (n=14). The first has been defined as subjects with an AHI ≥5 and ≤30 and the latter as subjects with an AHI >30 (1).
The following clinical data were recorded: the Epworth Sleepiness Scale and the body mass index (BMI).
Polysomnography
Overnight PSG was performed using DeltaMed (France, Coherence 4 NT) and Nihon Kohden (Japan, 2011) for PSG performed after 2012. Sleep states were assessed by recording biopotentials (electroencephalogram, electromyogram, electrooculogram), qualitative recordings of respiratory effort (piezo sensors), airflow (thermal sensors), and oxygen saturation (pulse oxymetry). The sampling frequency for the equipment DeltaMed was 256 Hz and 500 Hz for Nihon Kohden.
Respiratory events were scored as follows: apnea has been defined as a cessation of the airflow for more than 10 sec. Hypopneas have been defined as a reduction of more than 50% of the oro-nasal flow amplitude during 10 sec, accompanied by >3% desaturation and/or arousal. Hypopneas are classified as obstructive if there is evidence of upper airway resistance such as snoring, paradoxical motion in the respiratory bands, or inspiratory flow limitation on nasal pressure signal (1).
Polysomnographic scoring and staging was based on Rechtschaffen and Kales study, and episodes of arousals were assessed according to the guidelines in the previous studies (19).
HRV analysis
To study the HRV in different cycles, periods of 5 min have been selected from REM sleep and NREM sleep stages (represented by stage sleep 2 and slow wave sleep) in each third of sleep period. Transitions between sleep stages accompanied by sympathetic activation have been avoided (20).
For each selected segment, the occurrence of obstructive respiratory events has been noted. The arousals have been avoided (9). The selected segments of the ECG have been extracted and converted into text files.
Electrocardiographic signals acquired by the polysomnographic machine were digitalized by the sampling rate of 256 Hz for DeltaMed equipment and 500 Hz for Nihon Kohden equipment. The analysis of HRV was performed by the Kubios HRV (version 2.1, Finland) software after research and correction of artifacts, in accordance with the guidelines issued by the European Society of Cardiology and The North American Society of Pacing and Electrophysiology in 1996 (6).
Analyzed time-domain variables were mean RR and RMSSD. Mean RR is the mean of RR intervals. RMSSD is the square root of the mean of the sum of the squares of differences between adjacent RR intervals. In frequency-domain analysis, the power was calculated for very-low-frequency (VLF, 0.0033–0.04 Hz), low-frequency (LF, 0.04–0.15 Hz), and high-frequency bands (HF, 0.15–0.40 Hz). The LF/HF ratio was also included in the statistics. Normalized values of HF (nuHF) and LF (nuLF) bands have been re-calculated using the formulas of nuLF=LF/HF+LF and nuHF=HF/HF+LF.
Statistical analysis
Results were expressed as means (SD). When variables showed a normal distribution, a one-way ANOVA was performed, followed by a post hoc test. When variables were not normally distributed, a non-parametric test for independent samples was performed, followed by a Mann-Whitney U test. Bivariate correlations were estimated with Pearson or Spearman coefficients, as appropriate. A value of p<0.05 two-tailed has been considered significant with 95% confidence intervals.
Results
Clinical and PSG characteristics
The differences of mean age (38±9.4 vs. 42±7 years) and BMI (29±5 vs. 30±5 kg/m) were not significant between the mild/moderate and the severe OSA groups. As indicated in Table 1, the two groups were comparable as far as total sleep time, sleep efficiency, and the percentage of sleep stage 2 are concerned. Compared with the mild/moderate group, the severe OSA group had a higher stage 1 sleep, REM sleep percentage, a higher arousal index, and a lower mean oxyhaemoglobin saturation as determined by pulse oxymetry (SpO2).
Table 1.
Polysomnographic data
| Variable | Mild/Moderate OSA (n=16) Mean±SD | Severe OSA (n=14) Mean±SD | p |
|---|---|---|---|
| SPT (min) | 417±52.4 | 426.65±76.27 | >0.05 |
| SE (%) | 83.69±6.62 | 78.1±9.27 | >0.05 |
| SL (min) | 13.28±6.44 | 11.10±7 | >0.05 |
| Arl (total) | 8.67±6 | 15.7±8.22 | 0.015 |
| S1 (%) | 11.26±3.8 | 16.5±9.6 | 0.03 |
| S2 (%) | 53.3±7.05 | 58.53±9.3 | >0.05 |
| S3+S4 (%) | 16.86±5.54 | 9.4±10.5 | 0.02 |
| REM (%) | 18.34±3.6 | 15.2±8 | 0.02 |
| Average SpO2 | 95.4±2.09 | 89.3±3.05 | 0.02 |
| ODI | 20.6±7.2 | 56.63±22.2 | 0.02 |
OSA, obstructive sleep apnea; SPT, sleep period time; SL, sleep latency; SE, sleep efficiency; S1, stage 1 sleep; S2, stage 2 sleep; S3, stage 3 sleep; S4, stage 4 sleep; REM, REM sleep stage; AHI, apnea–hypopnea index; ODI, oxygen desaturation event index; ArI, arousal index.
HRV analysis during sleep stages
In order to determine whether the heart rate autonomic control differs significantly from wake base line for each sleep stage, HRV indices for each sleep stage have been compared to their corresponding wake and other sleep stage measures.
Table 2 shows that with all participants pooled together, during S2, the mean RR increased in relation to the wake stage accompanied with a significant increase of HF and HFnu and a decrease of LFnu and LF/HF ratio compared to REM sleep, the fact that reflects a parasympathetic activation and sympathetic inhibition during NREM sleep.
Table 2.
Comparison of HRV indices during sleep stages and wake
| All participants | Low AHI | High AHI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Stage | n | Value (Mean±SD) | p | Multiple comparisona | n | Value (Mean±SD) | p | Multiple comparisona | n | Value (Mean±SD) | p | Multiple comparisona | |
| Mean RR | W | 22 | 935.62±15 | 0.03 | W>S2 | 13 | 903±148.5 | 0.03 | S2>W | 9 | 797.9±11 | 0.03 | S2>W |
| S2 | 73 | 975.65±15 | REM>W | 38 | 998.67±16 | 35 | 950.58±133.7 | REM>W | |||||
| SWS | 30 | 942.08±14 | 19 | 956.41±17 | 11 | 899.8±100.6 | |||||||
| REM | 103 | 860±14 | 57 | 969.6±162 | 46 | 907.99±98 | |||||||
| RMSSD | W | 22 | 63.53±43 | >0.05 | 13 | 52.5±31.1 | >0.05 | 9 | 48.95±43 | >0.05 | |||
| S2 | 73 | 60.39±43 | 38 | 54.45±39 | 35 | 73.27±45.63 | |||||||
| SWS | 30 | 65.16±45 | 19 | 60.64±513 | 11 | 59.97±26.29 | |||||||
| REM | 103 | 51.02±35 | 57 | 59.72±41.9 | 46 | 71.9±48.21 | |||||||
| LF | W | 22 | 45.57±17 | >0.05 | 13 | 47.24±17.7 | >0.05 | 9 | 42.31±24 | >0.05 | |||
| S2 | 73 | 44.99±17 | 38 | 41.88±17 | 35 | 49.58±16.38 | |||||||
| SWS | 30 | 50.47±15.7 | 19 | 45.5±19.68 | 11 | 44.01±12.65 | |||||||
| REM | 103 | 45.22±20.2 | 57 | 50.21±15.4 | 46 | 50.79±16.3 | |||||||
| HF | W | 22 | 42.64±20 | 0.02 | S2>REM | 13 | 40.7±17.16 | 0.01 | S2>REM | 9 | 49.89±29 | >0.05 | |
| S2 | 73 | 44.31±21.2 | 38 | 48.37±20 | 35 | 36.41±20.22 | |||||||
| SWS | 30 | 34.50±19.1 | 19 | 47.17±22.8 | 11 | 39.36±17.83 | |||||||
| REM | 103 | 44.46±22.7 | 57 | 35.79±18.7 | 46 | 32.9±19.8 | |||||||
| LFnu | W | 22 | 52.71±20 | 0.018 | REM>S2 | 13 | 53.19±19.2 | 0.02 | S2<REM | 9 | 47.36±28 | >0.05 | |
| S2 | 73 | 51.71±20.3 | 38 | 47.01±20 | 35 | 58.91±19.98 | |||||||
| SWS | 30 | 60.69±19.1 | 19 | 49.84±22.6 | 11 | 54.95±16.19 | |||||||
| REM | 103 | 50.81±23.2 | 57 | 59.43±19.1 | 46 | 62.25±19.2 | |||||||
| HFnu | W | 22 | 47.18±20 | 0.018 | S2>REM | 13 | 46.27±20.4 | 0.02 | S2>REM | 9 | 47.36±28 | >0.05 | |
| S2 | 73 | 48.22±2.3 | 38 | 52.9±20.0 | 35 | 40.97±19.97 | |||||||
| SWS | 30 | 39.21±19 | 19 | 50.09±22.5 | 11 | 44.98±16.18 | |||||||
| REM | 103 | 49.06±23.2 | 57 | 40.48±19.0 | 46 | 37.63±19.2 | |||||||
| LF/HF | W | 22 | 2.07±0.4 | 0.018 | REM>S2 | 13 | 1.62±0.8 | 0.02 | REM>S2 | 9 | 1.87±0.6 | >0.05 | |
| S2 | 73 | 1.64±0.3 | REM>SWS | 38 | 1.62±0.5 | 35 | 2.56±0.5 | ||||||
| SWS | 30 | 2.84±0.5 | 19 | 1.67±0.38 | 11 | 2.87±0.4 | |||||||
| REM | 103 | 1.72±0.4 | 57 | 2.71±0.6 | 46 | 3.01±0.5 | |||||||
HRV indices for each sleep stage were compared to their corresponding wake and other sleep stage measures by ANOVA test followed by post hoc test, when variables were normally distributed. When variables were not normally distributed, a non-parametric test for independent samples was performed, followed by a Mann-Whitney U test.
No significant difference has been observed in the high AHI group except for the mean RR which increased significantly during NREM sleep compared to the wake stage.
Compared to mild/moderate group, the high AHI group showed a significantly higher LFnu and LF/HF during S2 (p=0.013, p=0.008). The HFnu and HF were significantly lower in the severe OSA group during S2 (p=0.013). During SP, the mean RR was higher in mild/moderate group (p=0.026). These results indicated a significant cardiac deceleration during sleep stages in the mild/moderate OSA group compared to high AHI group.
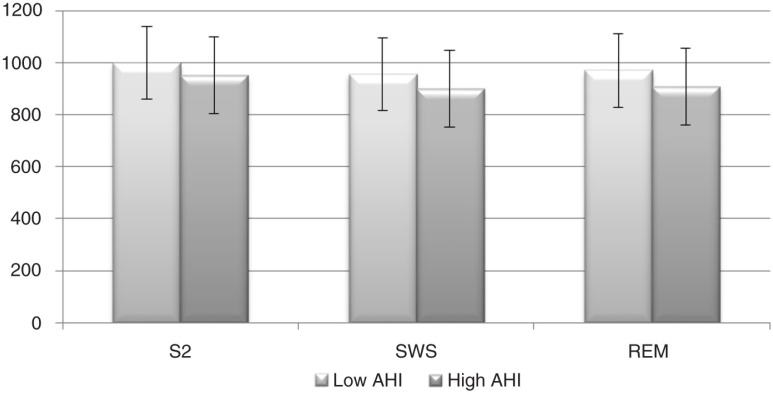
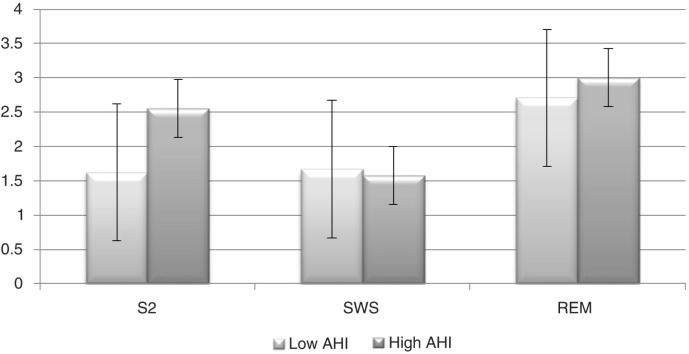
Figures 1 and 2 illustrate the profile of mean RR and the LF/HF ratio along sleep stages.
Fig. 1.
Mean of the RR intervals (mean RR) profile across sleep stages. Error bars represent±standard error of the mean. S2, sleep stage 2; SWS, slow wave sleep; REM, rapid-eye movement sleep; AHI, apnea–hypopnea index.
Fig. 2.
The low frequency/high frequency (LF/HF) ratio profile across sleep stages. Error bars represent±standard error of the mean. S2, sleep stage 2; SWS, slow wave sleep; REM, rapid-eye movement sleep; AHI, apnea–hypopnea index.
The circadian effect
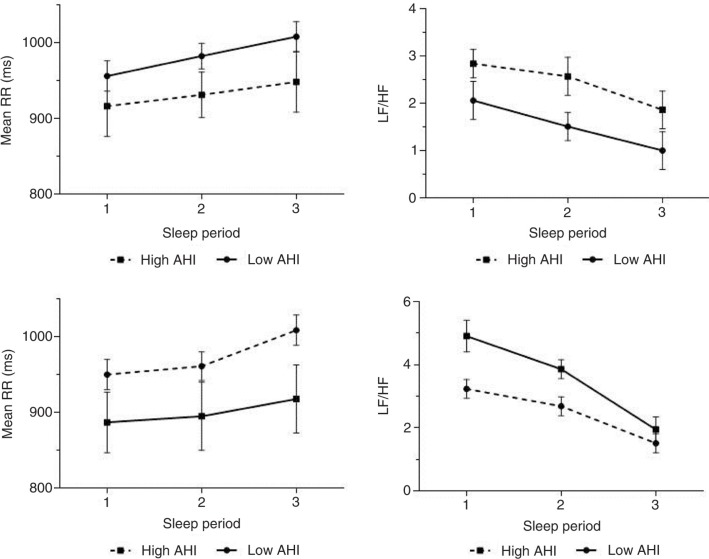
To study the variation of HRV indices along the night, the sleep period has been divided into three parts. During NREM sleep, the parasympathetic activity tends to increase during the night (Fig. 3a and b). But it was not significant. The same result has been observed during REM sleep (Fig. 3c and d).
Fig. 3.
Profiles of the mean of the RR intervals (mean RR) and the low frequency/high frequency (LF/HF) ratio for the different parts of sleep period during NREM sleep (a, b) and REM sleep (c, d). Error bars represent±standard error of the mean.
Apnea effect
By comparing the periods of 5 min, which are marked by the occurrence of respiratory events, with those without any obstructive respiratory event, we have found that the RMSSD was significantly lower in the absence of events (Table 3).
Table 3.
Comparison of HRV during free-event periods to those with obstructive events
| Free-event periods (n=74) | With event periods (n=123) | p | |
|---|---|---|---|
| Mean HR | 66.36±10.82 | 65.21±9.05 | 0.23 |
| Mean RR | 935.4±162.28 | 950.88±142.26 | 0.08 |
| RMSSD | 49.28±32 | 73.2±48.6 | 0.04 |
| LF | 47.24±18.63 | 47.94±15.86 | 0.4 |
| HF | 41.98±21.04 | 38.2±20.56 | 0.07 |
| LFnu | 53.61±21.5 | 57.17±20.06 | 0.08 |
| HFnu | 46.3±21.49 | 42.72±20.05 | 0.06 |
| LF/HF | 2.41±0.3 | 2.25±0.4 | 0.1 |
Correlation between the OSA severity and the HRV indices during sleep
Bivariate correlation has been estimated first. In the overall study group (n=30), there was a negative correlation between AHI and RR intervals (p=0.042, r=0.224), HF (p=0.001, r=−0.218) and HFnu (p=0.001, r=−0.226).
A positive correlation has been found for LFnu (p=0.001, r=0.225), LF (p=0.017, r=0.16) and LF/HF ratio (p=0.001, r=0.226).
After adjustment of age and BMI, the most significant correlation relationship found was for the mean RR (Table 4).
Table 4.
Partial correlation of AHI and HRV indices controlling age and body mass index of the subjects with obstructive sleep apnea
| Variable | p | r p |
|---|---|---|
| Mean RR | <0.0001 | −0.248 |
| LF | 0.024 | 0.144 |
| LFnu | 0.025 | 0.143 |
| HF | 0.037 | −0.133 |
| HFnu | 0.024 | −0.144 |
| LF/HF | 0.1 | 0.12 |
Discussion
The main aim of this study was to test the hypothesis that OSA was associated with an impaired cardiac autonomic modulation over the night. The second hypothesis was that HRV indices were affected by OSA severity.
Our results supported the first hypothesis. First, the mild/moderate OSA group presented a parasympathetic activation from wake to NREM sleep and a reversal to a balance of less parasympathetic modulation during REM sleep. This pattern is consistent with the findings of other studies performed in healthy subjects about the cardiac autonomic modulation along the sleep stages. These studies showed a reduced sympathetic outflow and increased parasympathetic modulation shift from wake to NREM sleep, a pattern reversed by entering in REM sleep (21–25).
The changes in the sympathovagal balance could be driven by oscillations in the metabolic demand during sleep, which markedly decreases during deep sleep and increases during REM sleep (26).
However, this shift of cardiac autonomic modulation is impaired among OSA severe group which showed no statistical difference between the sleep stages except for the mean RR. Our data suggest a more parasympathetic modulation reduction during sleep in the high AHI group. These findings are similar to those reported by other studies (27–29). Second, no statistically significant difference was found between the values of HRV indices across the night. However, the parasympathetic activity tended to increase with the progression of sleep during NREM and REM sleep associated to the decrease of sympathetic activity. These results suggest a circadian effect on the autonomic nervous system and a better quality of sleep reached progressively during the night. Several studies reported a circadian rhythm of heart rate with lower levels during the night compared to the day with a peak level at awakening (10, 30). This rhythm is driven by an endogenous central pacemaker located in the suprachiasmatic nucleus of the hypothalamus (10).
Our results are consistent with the findings of some studies performed during normal sleep which could not identify a clear sympathovagal modulation across the night (21, 25).
There are some data concerning the dynamic of HRV of OSA patients during sleep. Our findings are partially similar to those of Da Silva et al. (9) who showed a significant cardiac deceleration during the last quartile of the night, more pronounced in the high AHI group. A mechanism of parasympathetic overcompensation was evoked. This mechanism was associated with the increase of hydraulic pressure and the stimulation of carotid–aortic chemoreceptor in response to apnea (9).
The second hypothesis of this study was that HRV indices were correlated to the severity of OSA. A significant but small correlation was found for the frequency HRV parameters. Most of the temporal indices failed to show a significant correlation except for the mean RR. After control of age and BMI, the mean RR was found to be the most affected by AHI. Our results are partially similar to those of Zhu et al. (31) who found a negative correlation with the mean RR, suggesting it to be the best index to assess the sympathetic–parasympathetic balance over the night in OSA. However, no statistical difference was found for the spectral indices in their study. In some other previous studies, LF/HF was found to present the most significant correlation with the AHI (28, 32, 33).
Limitations
This study is one of few that have assessed the HRV by sleep stage and at the different parts of night with the presence of sleep apnea syndrome. However, it presents quite a few limitations. It is a retrospective study with a reduced sample. The interpretation of HRV parameters during the wake period may be biased due to the lack of control over environmental factors. The population study was based only on men, therefore not allowing a study of the differences according to gender. The choice of including men only has been previously explained.
One of the main limits of our study concerns measurement conditions of HRV during sleep.
The spectral study requires the respect of the signal stationary condition, which should vary little through time. Respecting this condition becomes an issue when studying parameters during sleep with the presence of OSA (14). Indeed, apnea induces tachycardia during arousal proceeding by reinforcement of respiratory sinus arrhythmia (14).
Measuring the HRV during this period does not respect the stationary condition. In spite of this, some authors have studied the effect of the respiratory events on HRV like Guilleminault et al. (34) who have found a significant increase of LF/HF and decrease of HF after the event.
Some other authors have chosen to study the HRV during the periods without respiratory events (28). This choice is also problematic because it is very hard for some patients to find free-event periods, without mentioning the risk of not scoring subclinical events (14).
The objective of this work was to study the modulation of the HRV parameters during different sleep stages at night aiming for studying the dynamics of the autonomous nervous system during the sleep cycles. It was difficult to find periods without respiratory events with a number of patients. These periods were included, while noting the onset of the obstructive events. The comparison of obstructive events with those without it shown in the RMSSD was significantly higher in the presence of respiratory event.
The increase of the RMSSD, which habitually testifies to the reinforcement of the parasympathetic nervous activity modulated by respiration, may be attributed to modifications in the cardiac rhythm that accompany these episodes. Its inability to distinguish between increasing HRV due to an erratic rhythm and an increase in parasympathetic activity makes it problematic and not reflective of the parasympathetic nervous system (14).
No other statistical difference was found including the mean RR. This may be attributed to the presence of no scored subclinical events.
Conclusion
This study is one of the few investigating the HRV by sleep stage at different parts of night with the presence of OSA. It shows the effects of OSA on sympathovagal modulation during sleep and this impact was correlated to the severity of the disease.
The mean RR seemed to be a better index allowing the sympathovagal balance appreciation during night in the OSA. Our findings could be used further to confirm the contribution of HRV parameters as a screening tool in the OSA.
Acknowledgements
We wish to thank Dr. Iheb BOUGMIZA for his help in the statistical study.
Conflict of interest and funding
The authors declare they have no conflicts of interest concerning this article.
References
- 1.Epstein LJ, Kristo D, Strollo PJ, Jr., Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108:246–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Pashayan AG, Passannante AN, Rock P. Pathophysiology of obstructive sleep apnea. Anesthesiol Clin North America. 2005;23:431–43. doi: 10.1016/j.atc.2005.02.004. vi. [DOI] [PubMed] [Google Scholar]
- 6.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–81. [PubMed] [Google Scholar]
- 7.Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. 2004;134:514–22. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- 8.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis risk in Communities. Circulation. 2000;102:1239–44. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 9.Da Silva SP, Hulce VD, Backs RW. Effects of obstructive sleep apnea on autonomic cardiac control during sleep. Sleep Breath. 2009;13:147–56. doi: 10.1007/s11325-008-0228-0. [DOI] [PubMed] [Google Scholar]
- 10.Boudreau P, Dumont G, Kin NM, Walker CD, Boivin DB. Correlation of heart rate variability and circadian markers in humans. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:681–2. doi: 10.1109/IEMBS.2011.6090153. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydin M, Altin R, Ozeren A, Kart L, Bilge M, Unalacak M. Cardiac autonomic activity in obstructive sleep apnea: time-dependent and spectral analysis of heart rate variability using 24-hour Holter electrocardiograms. Tex Heart Inst J. 2004;31:132–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Balachandran JS, Bakker JP, Rahangdale S, Yim-Yeh S, Mietus JE, Goldberger AL, et al. Effect of mild, asymptomatic obstructive sleep apnea on daytime heart rate variability and impedance cardiography measurements. Am J Cardiol. 2012;109:140–5. doi: 10.1016/j.amjcard.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Med Rev. 2012;6:47–66. doi: 10.1016/j.smrv.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Roche F, Gaspoz JM, Court-Fortune I, Minini P, Pichot V, Duverney D, et al. Screening of obstructive sleep apnea syndrome by heart rate variability analysis. Circulation. 1999;100:1411–15. doi: 10.1161/01.cir.100.13.1411. [DOI] [PubMed] [Google Scholar]
- 16.Raymond B, Cayton RM, Chappell MJ. Combined index of heart rate variability and oximetry in screening for the sleep apnoea/hypopnoea syndrome. J Sleep Res. 2003;12:53–61. doi: 10.1046/j.1365-2869.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- 17.Stein PK, Kleiger RE, Rottman JN. Differing effects of age on heart rate variability in men and women. Am J Cardiol. 1997;80:302–5. doi: 10.1016/s0002-9149(97)00350-0. [DOI] [PubMed] [Google Scholar]
- 18.Sato N, Miyake S, Akatsu J, Kumashiro M. Power spectral analysis of heart rate variability in healthy young women during the normal menstrual cycle. Psychosom Med. 1995;57:331–5. doi: 10.1097/00006842-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. Washington, DC: US Government Printing Office, US Public Health Service; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 20.Viola AU, Simon C, Ehrhart J, Geny B, Piquard F, Muzet A, et al. Sleep processes exert a predominant influence on the 24-h profile of heart rate variability. J Biol Rhythms. 2002;17:539–47. doi: 10.1177/0748730402238236. [DOI] [PubMed] [Google Scholar]
- 21.Cabiddu R, Cerutti S, Viardot G, Werner S, Bianchi AM. Modulation of the Sympatho-Vagal balance during sleep: frequency domain study of heart rate variability and respiration. Front Physiol. 2012;3:45. doi: 10.3389/fphys.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otzenberger H, Gronfier C, Simon C, Charloux A, Ehrhart J, Piquard F, et al. Dynamic heart rate variability: a tool for exploring sympathovagal balance continuously during sleep in men. Am J Physiol. 1998;275:H946–50. doi: 10.1152/ajpheart.1998.275.3.H946. [DOI] [PubMed] [Google Scholar]
- 23.Vanoli E, Adamson PB, Ba L, Pinna GD, Lazzara R, Orr WC. Heart rate variability during specific sleep stages. A comparison of healthy subjects with patients after myocardial infarction. Circulation. 1995;91:1918–22. doi: 10.1161/01.cir.91.7.1918. [DOI] [PubMed] [Google Scholar]
- 24.Zemaityte D, Varoneckas G, Sokolov E. Heart rhythm control during sleep. Psychophysiology. 1984;21:279–89. doi: 10.1111/j.1469-8986.1984.tb02935.x. [DOI] [PubMed] [Google Scholar]
- 25.Bonnet MH, Arand DL. Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr Clin Neurophysiol. 1997;102:390–6. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- 26.Wilde-Frenz J, Schulz H. Rate and distribution of body movements during sleep in humans. Percept Mot Skills. 1983;56:275–83. doi: 10.2466/pms.1983.56.1.275. [DOI] [PubMed] [Google Scholar]
- 27.Ueno LM, Drager LF, Rodrigues AC, Rondon MU, Mathias W, Jr., Krieger EM, et al. Day–night pattern of autonomic nervous system modulation in patients with heart failure with and without sleep apnea. Int J Cardiol. 2011;148:53–8. doi: 10.1016/j.ijcard.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds EB, Seda G, Ware JC, Vinik AI, Risk MR, Fishback NF. Autonomic function in sleep apnea patients: increased heart rate variability except during REM sleep in obese patients. Sleep Breath. 2007;11:53–60. doi: 10.1007/s11325-006-0083-9. [DOI] [PubMed] [Google Scholar]
- 29.Palma J-A, Iriarte J, Fernandez S, Valencia M, Alegre M, Artieda J, et al. Characterizing the phenotypes of obstructive sleep apnea: clinical, sleep, and autonomic features of obstructive sleep apnea with and without hypoxia. Clin Neurophysiol. 2014;125:1783–91. doi: 10.1016/j.clinph.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Elliott WJ. Cyclic and circadian variations in cardiovascular events*. Am J Hypertens. 2001;14(Suppl 6):291S–5S. doi: 10.1016/s0895-7061(01)02174-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhu K, Chemla D, Roisman G, Mao W, Bazizi S, Lefevre A, et al. Overnight heart rate variability in patients with obstructive sleep apnoea: a time and frequency domain study. Clin Exp Pharmacol Physiol. 2012;39:901–8. doi: 10.1111/1440-1681.12012. [DOI] [PubMed] [Google Scholar]
- 32.Park DH, Shin CJ, Hong SC, Yu J, Ryu SH, Kim EJ, et al. Correlation between the severity of obstructive sleep apnea and heart rate variability indices. J Korean Med Sci. 2008;23:226–31. doi: 10.3346/jkms.2008.23.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song MK, Ha JH, Ryu SH, Yu J, Park DH. The effect of aging and severity of sleep apnea on heart rate variability indices in obstructive sleep apnea syndrome. Psychiatry Investig. 2012;9:65–72. doi: 10.4306/pi.2012.9.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guilleminault C, Poyares D, Rosa A, Huang YS. Heart rate variability, sympathetic and vagal balance and EEG arousals in upper airway resistance and mild obstructive sleep apnea syndromes. Sleep Med. 2005;6:451–7. doi: 10.1016/j.sleep.2005.03.014. [DOI] [PubMed] [Google Scholar]