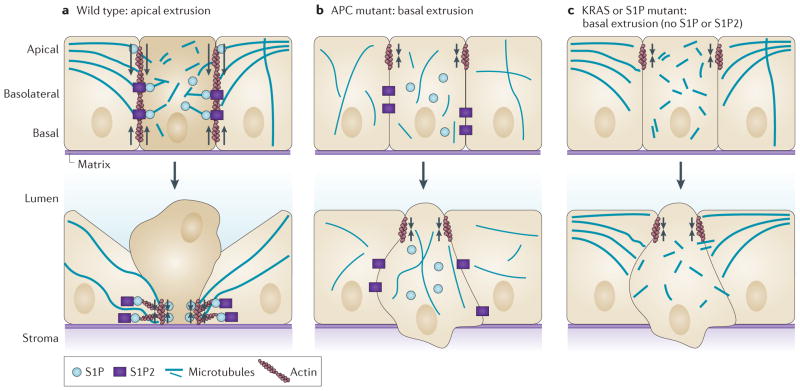
Figure 2. Modes of diverting extrusion basally.
a | During wild-type apical extrusion, the cell that is destined for extrusion (dark beige), as well as its neighbouring cells (light beige), reorient their microtubules to the basolateral interface. Reorientation of microtubules in the cell destined for extrusion is required for apical extrusion and presumably restricts the biologically active lipid sphingosine-1-phosphate (S1P) to the basolateral surface, where it binds to S1P receptor 2 (S1P2) expressed in the neighbouring cells to trigger apical extrusion. Microtubule reorientation in the neighbouring cells might reinforce RHO- mediated actomyosin contraction (arrows) at the basolateral surface. b | Mutations in the tumour suppressor adenomatous polyposis coli (APC) that disrupt microtubule dynamics can function cell-autonomously in the extruding cell to drive extrusion basally. Cell-autonomous apical contraction of cortical actin and myosin (arrows) at apical epithelial cell junctions can extrude the cell basally into the basement membrane. c | Oncogenic KRAS disrupts apical extrusion by downregulating both S1P and S1P2. Autophagy degrades S1P, and both S1P puncta and apical extrusion can be rescued by disrupting autophagy. Without apical extrusion signalling, junctional apical actin and myosin contraction (arrows) results in basal extrusion.