Figure 3. Treatment with Carboplatin +/− ABT888 improves survival in BRCA-mutant intracranial TNBC.
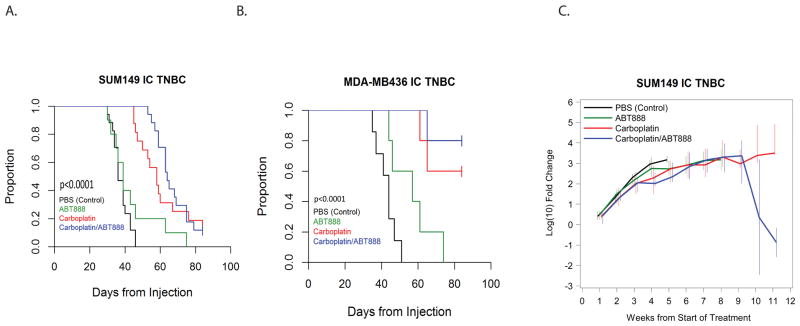
Animals in both models were treated with PBS (control), Carboplatin 50mg/kg/week (IP), ABT888 25mg/kg/day (OG) or combination Carboplatin+ABT888 (doses as in single agent therapy). Treatment began 14 days after intracranial implantation. A total of 4 animals in the SUM149 model and a total of 6 animals in the MDA-MB436 model treated with Carboplatin and Carboplatin+ABT888 were sacrificed 12 weeks post-intracranial implantation, as defined by IAUCUC protocol guidelines, and were censored for the purpose of the analysis.
A. Median survival of the SUM149 intracranial model.
B. Median survival of the MDA-MB-436 intracranial model.
C. Dynamic changes in intracranial tumor growth measured by median fold change of the bioluminescence signal intensity from the start of the treatment in SUM149 TNBC murine model. The median log fold changes for each treatment group were calculated weekly. Data for each time point were plotted if at least 2 animals per treatment group remained alive. The vertical bars represent the interquartile ranges (25th–75th percentiles).
