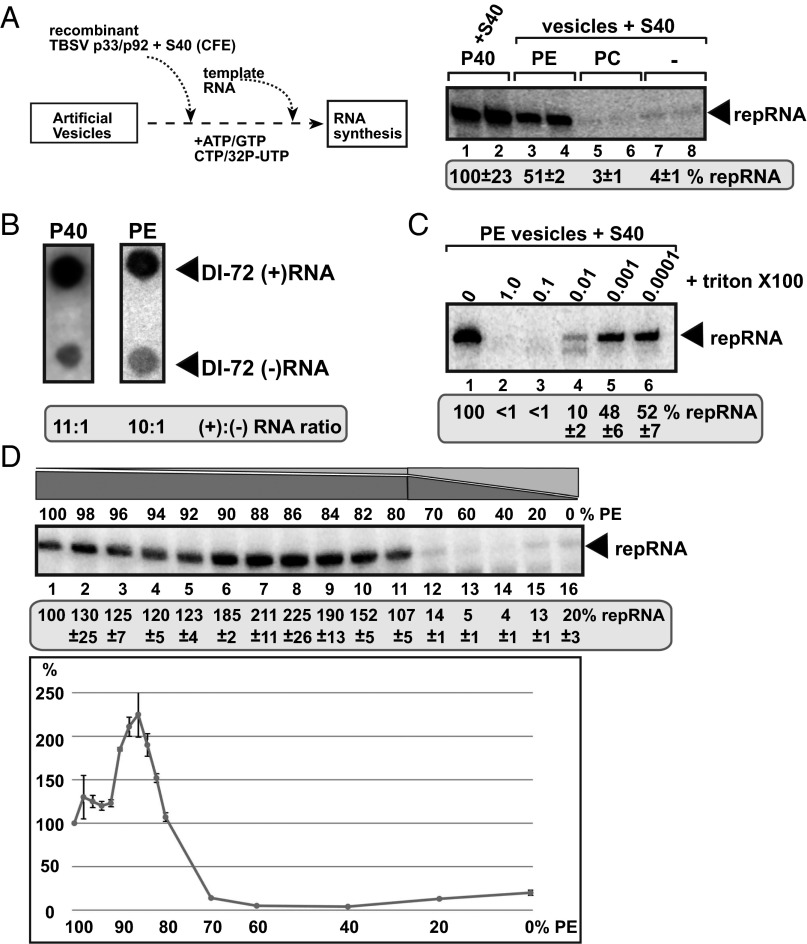
Fig. 1.
In vitro reconstitution of the TBSV replicase in artificial PE vesicles. (A) Scheme of the replicase assembly assay. Purified recombinant p33 and p92pol replication proteins of TBSV in combination with the TBSV-derived (+)repRNA were added to PE or PC vesicles or the P40 membrane fraction of yeast CFE. The S40 fraction of CFE was also added to each sample to provide soluble host factors required for TBSV VRC assembly. The denaturing PAGE analysis of the 32P-labeled repRNA products obtained is shown. The full-length repRNA is denoted by an arrow. The CFE-based replication assay was chosen as 100% (lanes 1 and 2). (B) Asymmetrical RNA synthesis by TBSV VRCs assembled in PE vesicles. The amounts of TBSV (+)- and (–)-stranded RNA products produced by the reconstituted TBSV VRCs are measured by using the 32P-labeled repRNA probes generated in the in vitro assays. The blot contains the same amount of cold (+)- and (–)-stranded DI-72 RNA. (C) TBSV RNA synthesis by the reconstituted VRCs in PE vesicles requires vesicles/membrane bilayers. The PE vesicles were disrupted by Triton-X100 treatment as shown. The denaturing PAGE analysis of the replicase products is as in panel A. (D) Increased VRC activity in PE vesicles containing a fraction of other phospholipids. The PE vesicles contained the shown percentage of PE plus a mixture of other phospholipids (the ratio in the phospholipid mix was 54.5 of PC, 6.1 of PI, 1.2 of PS, 8.3 of PG, 0.9 of LysoPE, and 0.6 of LysoPC), which was based on their ratios in TBSV-infected N. benthamiana leaves. The denaturing PAGE analysis of the replicase products is as in panel A.