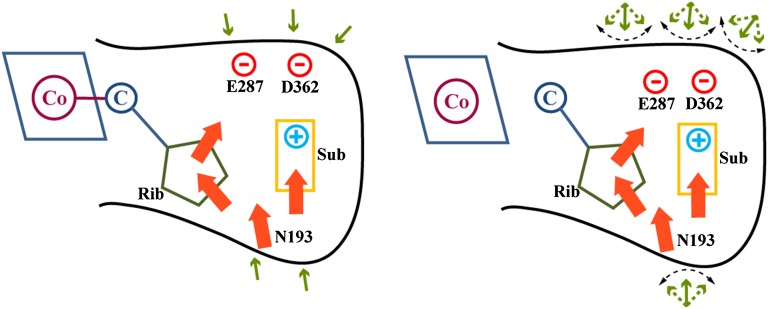
Fig. 5.
A schematic description of the origin of the entropic effect. The figure describes the situation in EAL by considering schematically the interactions between the leaving-group ribose (rib) plus the substrate (sub) and their first shell (explicit details are given in Fig. S5), while showing the response of the second solvation shell (the group outside the black solid line). The motion of the TS or state III results in stronger electrostatic interactions within the groups inside the solid black line and thus weaker interaction between theses groups and the polar groups in the second solvation shell. Thus, the bond-breaking process that involves an increase of the electrostatic interaction within the solid black line leads to weaker interaction with the second solvation shell, which starts to experience less polar solute, and is free to fluctuate more, thereby generating a larger entropic effect.