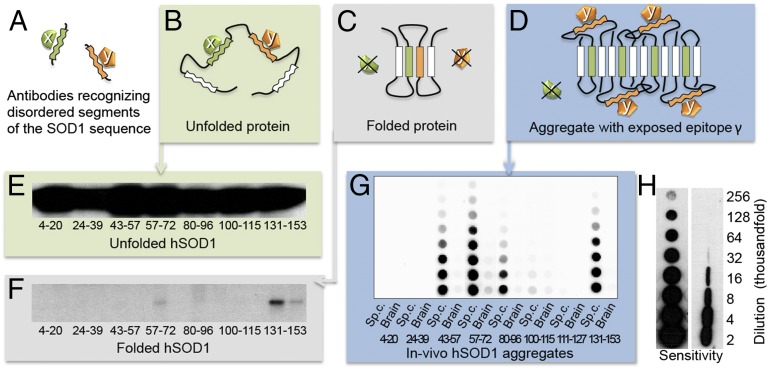
Fig. 2.
Discrimination between “disordered” and “structured” sequence segments by the binary epitope-mapping assay. (A) Antibodies (x and y) were raised against short, consecutive segments of the hSOD1 sequence (Fig. 1). (B) In the globally unfolded state of hSOD1, the individual sequence epitopes are flexible and can adapt to the antigen-binding sites of the anti-peptide antibodies. (C) In the rigid folded hSOD1, the sequence epitopes adopt a fixed structure incompatible with antibody binding. (D) In partially ordered hSOD1 aggregates, antibody x cannot bind to its sequence epitope because this is ordered/hidden in the aggregate core, whereas antibody y can, because the epitope protrudes freely from the aggregate surface. (E) Western blot of hSOD1 captured by immobilized anti-peptide antibodies incubated with unfolded/denatured hSOD1 in solution. All of the antibodies bind, showing full exposure of flexible sequence segments in Fig. 1D. (F) Corresponding data for natively folded hSOD1, giving no binding because the sequence epitopes are now structured/hidden. Two sequential incubations were carried out, the first to capture any traces of disordered hSOD1 present in the preparation. E and F reproduced from ref. 9. (G) Aggregates in spinal cord and brain from a terminally ill hSOD1G93A mouse captured on a filter in a dot-blot apparatus and stained with the anti-peptide antibodies, showing the binary fingerprint of disordered and structured/hidden sequence regions of the constituent hSOD1 monomers. (H) The benchmarking 57–72 antibody detects hSOD1 in filter-captured aggregates with 10-fold higher sensitivity than hSOD1 restricted on a Western immunoblot membrane (SI Materials and Methods). The figures indicate the degree of dilution of the spinal-cord tissue.