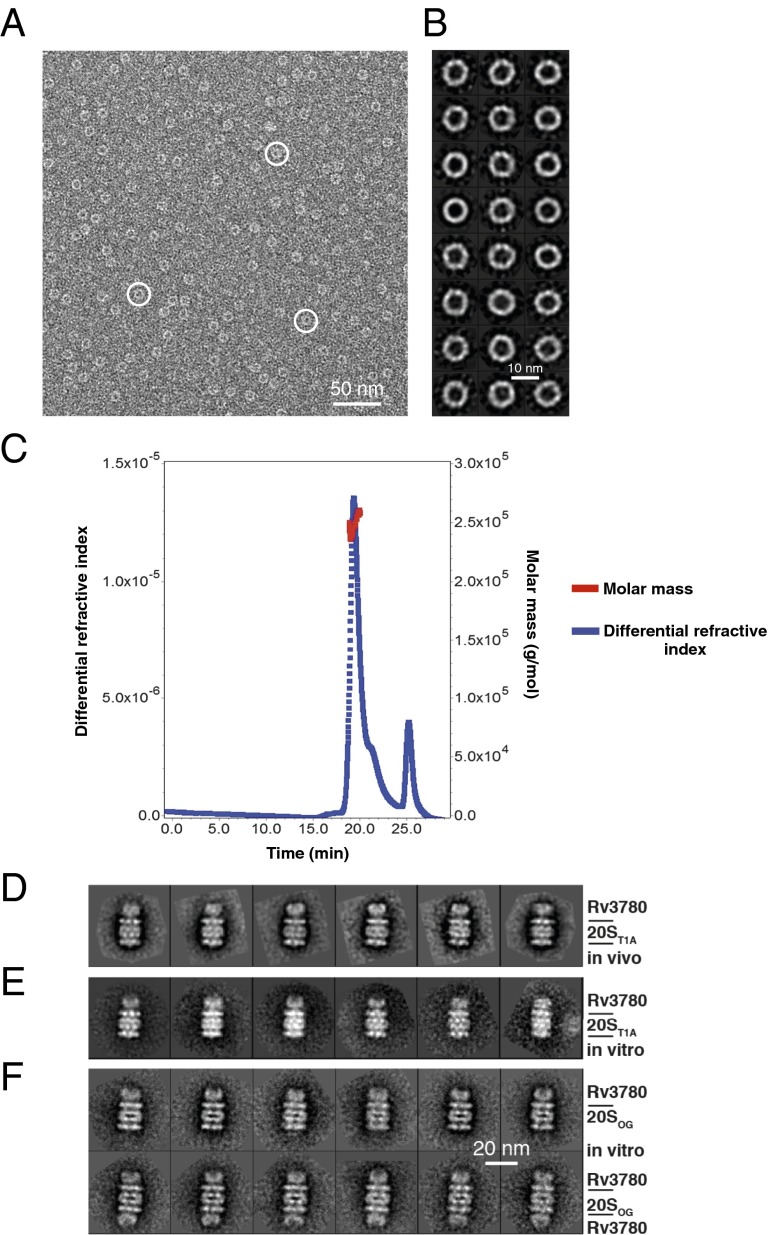
Fig. 2.
Rv3780 forms oligomeric rings and caps 20S CPs. (A) A raw electron micrograph of negatively stained His6–Rv3780 particles expressed and purified as a recombinant protein from E. coli. White circles mark three Rv3780 rings. (B) Shown are 24 representative reference-free 2D class averages of His6–Rv3780 ring structure. (C) Recombinant purified His6–Rv3780 was separated by SEC and analyzed by MALS. (D) Six representative reference-free 2D class averages of presumptive endogenous Rv3780 rings capping one end of TAP-tagged 20ST1A CP purified from an M. tuberculosis mpa mutant. (E) Six representative reference-free 2D class averages of an in vitro reconstituted Rv3780–20S CP complex with both M. tuberculosis His6–Rv3780 and 20ST1A CP–His6 proteins purified from E. coli. (F) Selected reference-free class averages of the singly capped (Upper) and doubly capped (Lower) Rv3780–20SOG CP complex reconstituted in vitro by incubating M. tuberculosis His6-tagged proteins purified from E. coli in the presence of bortezomib. D–F are on the same scale.