Figure 8.
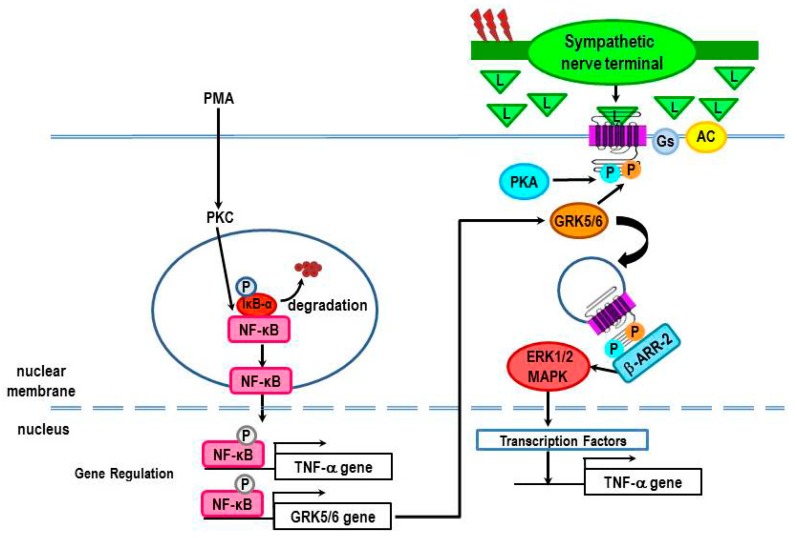
The presence of a β2-AR ligand during lipopolysaccharide (LPS) or phorbol 12-myristate 13-acetate (PMA)-induced macrophage activation causes reduced or increased production of TNF-α, IL-12 and nitric oxide respectively. The β2-AR-induced immunosuppression in LPS-treated macrophages is consistent with the activation of adenylyl cyclase (AC), and subsequently PKA. LPS promotes adenylyl cyclase activity, suppresses translocation of GRK2 to the membrane, and reduces the expression of GRKs 5 and 6. These LPS-induced effects are expected to increase β2-AR signaling via the cAMP-PKA canonical pathway. In contrast, the β2-AR-induced immune enhancement in PMA-treated macrophages is not consistent with receptor activation of the cAMP-PKA pathway. Instead, we propose that the responsible mechanism is a shift in receptor signaling from the canonical to the non-canonical pathway.