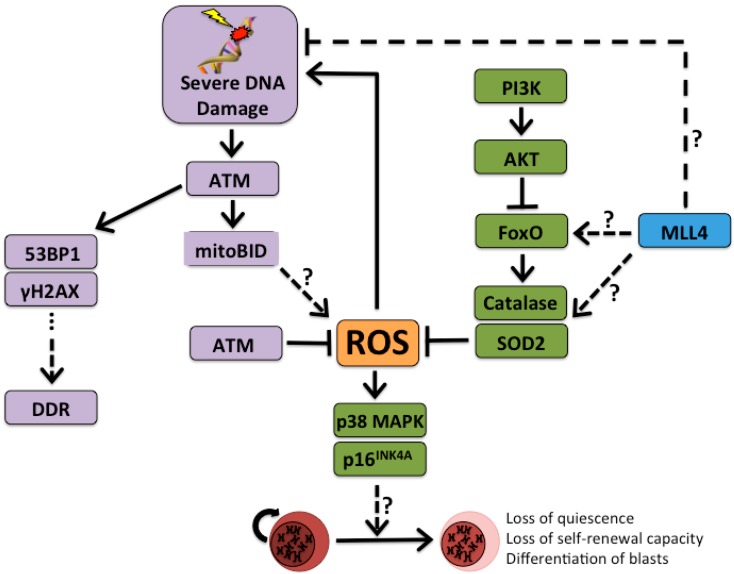
Figure 1.
The ROS rheostat of hematopoietic stem cell (HSC) maintenance. Accumulation of DNA damage and genotoxic oxidative stress contributes to a common pathway that leads to loss of self-renewal capacity of HSCs and leads HSCs to exit their quiescent state. This contributes to the gradual decline of functional HSCs in the bone marrow. Mixed lineage leukemia 4 (MLL4) activates forkhead box O (FoxO) targets through an unknown mechanism, and MLL4 expression is shown to be protective in the MLL1-AF9 (ALL1-fused gene from chromosome 9, or MLLT3) of AML by reducing the accumulation of ROS and, thus, DNA damage. Mll4-deficiency may also contribute to DNA damage through a ROS-independent mechanism. DNA damage results in the activation of ATM and, subsequently, DDR. Accumulation of γH2AX and co-localization with 53BP1 serve as markers of DDR, as in Flach, et al. Under normal conditions, ATM helps to maintain ROS at low levels. However, in the face of severe DNA damage ATM contributes to the accumulation of ROS and loss of quiescence in HSCs. ATM, ataxia telangiectasia mutated; FoxO, forkhead box O; DDR, DNA damage response; γH2AX, phosphorylated histone H2AX; MLL4, mixed-lineage leukemia 4; mitoBID, mitochondrial BH3 interacting-domain death agonist; MLL4, mixed-lineage leukemia 4; p38 MAPK, p38 mitogen-activated protein kinases; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; SOD2, superoxide dismutase 2; TP53BP1, tumor suppressor p53-binding protein 1. p16INK4A, cyclin dependent kinase inhibitor 2A; AKT, protein kinase 3. Solid arrows represent known mechanisms; dashed arrows labeled with question marks represent unknown mechanisms.