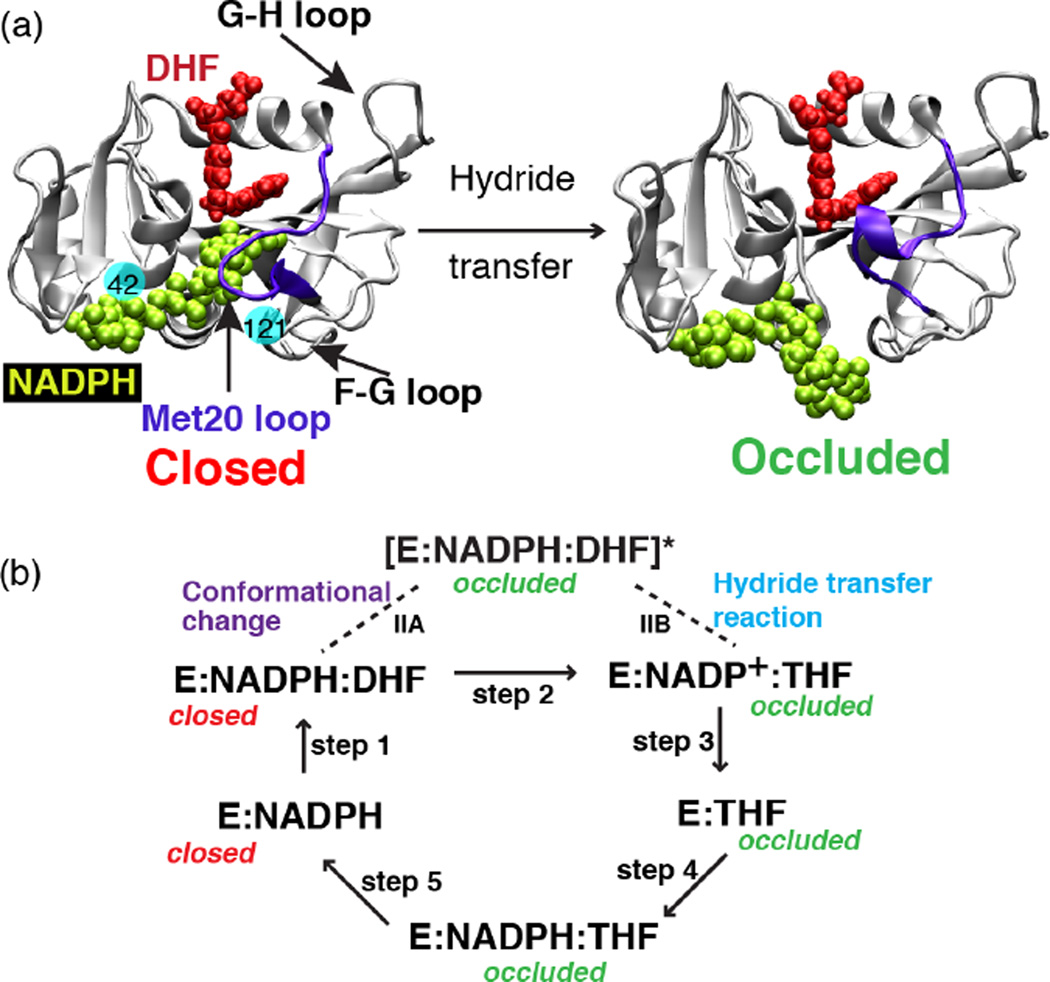
Fig. 1.
The structure of E. coli DHFR and the conformational changes that take place during the catalytic process. (a) Crystal structure of DHFR in the closed (PDB ID: 1RX2) and the occluded (PDB ID: 1RX6) states. In the closed state, the Met20 loop stacks against the nicotinamide ring of the cofactor (NADPH) while in the occluded state the loop prevents cofactor from accessing the active site pocket. Solid balls illustrate the locations of mutated residues 42 and 121. (b) Schematic representation of the catalytic pathway of DHFR as deduced from several kinetic and structural studies. In the holoenzyme E:NADPH and the Michaelis Menten complex E:NADPH:DHF, the Met20 loop adopts the closed conformation. In the three product complexes, E:NADP+, E:THF and E:NADPH:THF, the Met20 loop occurs in the occluded conformation. We have performed simulations capturing the enzyme’s transition between the closed and occluded state (step IIA, above). (Fig. 1 of Arora K and Brooks, C. L. (2009), J. Amer. Chem. Soc. 131:5642, Copyright © 2007, The American Chemical Society.