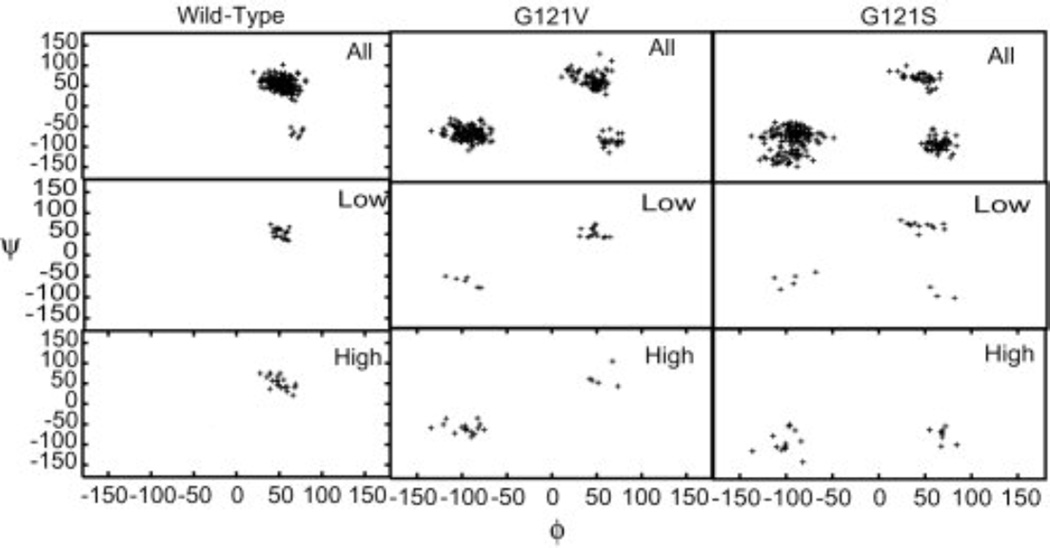
Fig. 6.
Backbone ϕ/ψ angles for glutamate 17. The first row in each panel corresponds to the entire trajectory, while the second and third rows correspond to structures giving rise to low and high transfer barriers, respectively. Dihedral angles are exhibited by the mutant proteins that are not observed in wild type DHFR. These alternate dihedral values are generally associated with higher barriers. Glutamate 17 is observed to be primarily in the region ϕ 50°/ψ 50° for the wild type protein and for the low-barrier structures of the two mutants. Structures giving rise to higher barriers in the mutant proteins are found in the vicinity of ϕ −100°/ψ −50°. (Figure 5 of Thorpe IF, Brooks III CL (2004) Proteins: Struct. Funct. Bioinf. 57: 444, Copyright 2004, © The Protein Society)