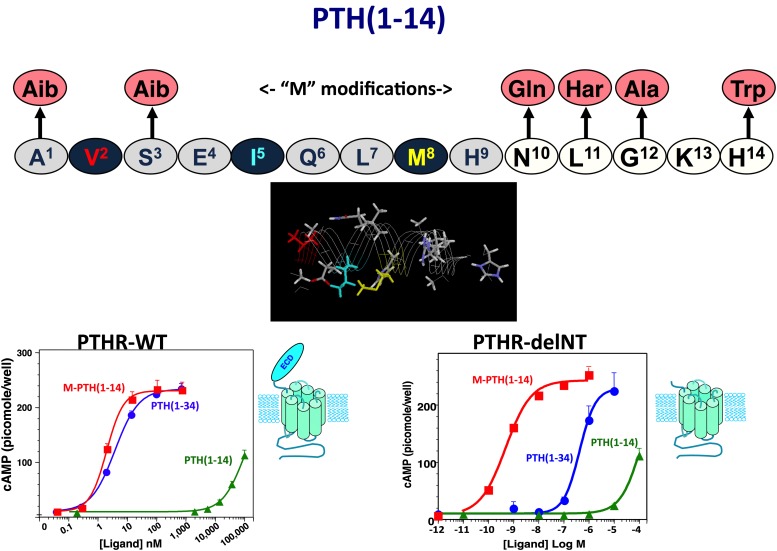
Fig. 4.
Optimized PTH(1–14) domain. Shown is the sequence of the human PTH(1–14) peptide and the six amino acid substitutions that comprise the “M” set of modifications that together increase cAMP signaling potency of the PTH(1–14) fragment by five orders of magnitude and stabilize α-helical structure in the otherwise disordered PTH(1–14) peptide (Tsomaia et al., 2004). The graphs depict dose-response curves for cAMP generation obtained for PTH(1–34), M-PTH(1–14), and native PTH(1–14) in COS-7 cells transiently transfected to express either the intact wild-type PTHR1 (left) or the PTHR1-delNT construct (right), which is deleted for most of the ECD. These data reveal that whereas potency of PTH(1–34) is about 100-fold weaker on PTHR1-delNT than on the wild-type PTHR1, the PTH(1–14) fragments exhibit the same potency on the two receptors. The data thus demonstrate that whereas PTH(1–34) requires both the ECD and the TMD regions to obtain full potency, the PTH(1–14) portion of the ligand only interacts with the TMD region of the receptor (Shimizu et al., 2001b).