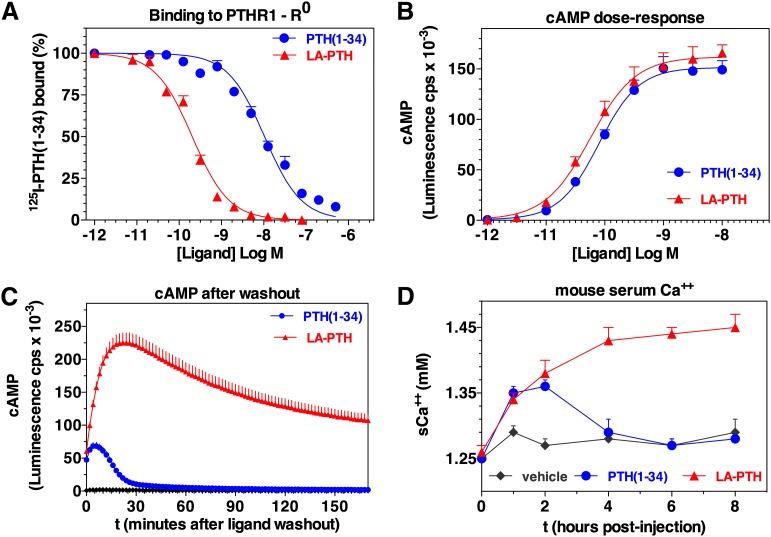
Fig. 9.
Properties of a long-acting PTH analog. Shown is the capacity of the long-acting analog, LA-PTH, a hybrid peptide consisting of a modified M-PTH(1–14) segment joined to a modified PTHrP(15–36) segment, to bind with high affinity to the R0 PTHR1 conformation and thus mediate prolonged signaling responses in cells and in animals. The analog is compared with PTH(1–34) for (A) binding to the PTHR1 in the G protein–independent conformation, R0; (B) inducing dose-dependent cAMP signaling responses in HEK-293 cells expressing the rat PTHR1 and the luciferase-based glosensor cAMP reporter; (C) capacity to maintain cAMP responses in the same cells after an initial application of ligand (0.3 nM/15 minutes) and then a washout (at t = 0') of unbound ligand; and (D) stimulating blood ionized calcium responses in mice [after injections with either vehicle, PTH(1–34) at 50 nmol/kg or LA-PTH at 10 nmol/kg]. Not shown is that LA-PTH bound to the G protein–coupled PTHR1 conformation, RG, with an affinity only threefold higher than that of PTH(1–34); this similar affinity for RG likely explains the similar potencies observed in the cAMP dose-response assays, whereas the greater difference in binding to the R0 PTHR1 conformation likely explains the more prolonged cAMP responses in cells and calcemic responses in animals. Adapted from Maeda et al. (2013).