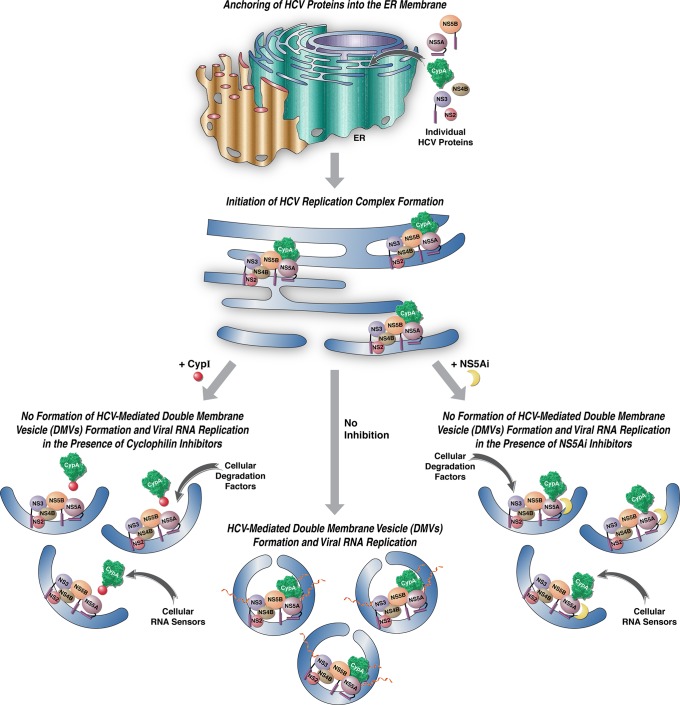
FIG 5.
Models for the MoA of CypI and NS5Ai on HCV-mediated DMV formation and viral RNA replication. In the middle model, in the absence of inhibitors, HCV components accumulate at the ER via their membrane anchor domains and initiate the formation of enzymatically active replication complexes (RCs) within newly formed protective DMVs. In the left model, due to the binding of CypI to the isomerase pocket of CypA, the host isomerase protein is incapable of interacting with the domain II of NS5A during RC formation. Without a proper folding by CypA, NS5A is unable to drive DMV formation, where HCV RNA synthesis and replication normally occur in a shielded compartment, leading to an abortive replication. In the right model, due to the binding of NS5Ai to the domain I of NS5A, the viral protein for a still undefined reason is unable to drive DMV formation. In the absence of a protective cellular compartment, RCs, especially viral RNA, are completely exposed to cellular sensors of foreign RNA, as well as to cellular-degrading factors of viral components.