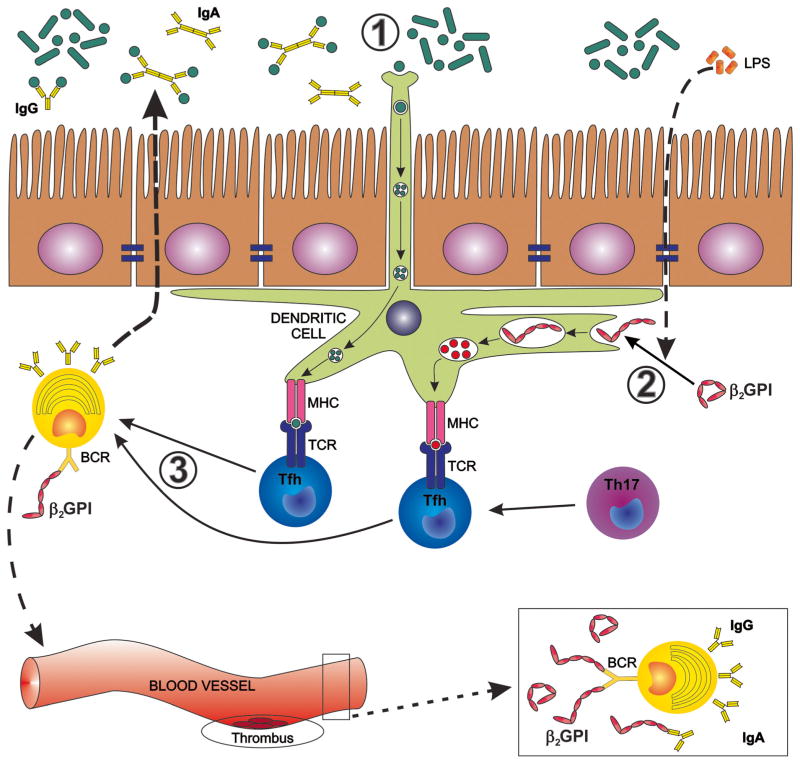
Fig. 1.
Proposed influence of the gut microbiota on induction and maintenance of anti-β2-glycoprotein I antibodies (anti-β2GPIs). In genetically predisposed individuals, the gut microbiota may drive the induction of anti-β2GPI antibodies by several mechanisms that can occur separately or in combination. 1 Cross-reactive gut commensal antigens are recognized by mucosal dendritic cells (DCs) sampling the intestinal lumen or by phagocytosis after barrier disruption or apoptosis of intestinal epithelial cells (not shown). 2 Commensal-derived LPS, phospholipids, and oxidative stress lead to a conformational change of β2GPI that exposes cryptic epitopes in domains I and V of β2GPI. DCs and other antigen-presenting cells take up unfolded β2GPI bound to LPS or phospholipids via receptors that bind these complexes, e.g., Toll-like receptors. 3 DCs present cross-reactive commensal antigens, cryptic β2GPI antigens, or both in an HLA class II-restricted manner to cognate CD4+ helper T cells in secondary lymphoid organs. CD4+ helper Tcell subsets assist antigen-specific B cells via CD40 ligand and other co-stimulatory receptors (not shown). These B cells then secrete IgA/IgG in the mucosal lumen and in the systemic circulation, respectively. The helper T cells leading to IgA/IgG production are follicular helper Tcells (Tfh) or, as shown in the gut, also ex-Th17 cells that convert to Tfh-like cells [62•]. A “second hit” in the vasculature then leads to thrombotic events as detailed in the main text [41].