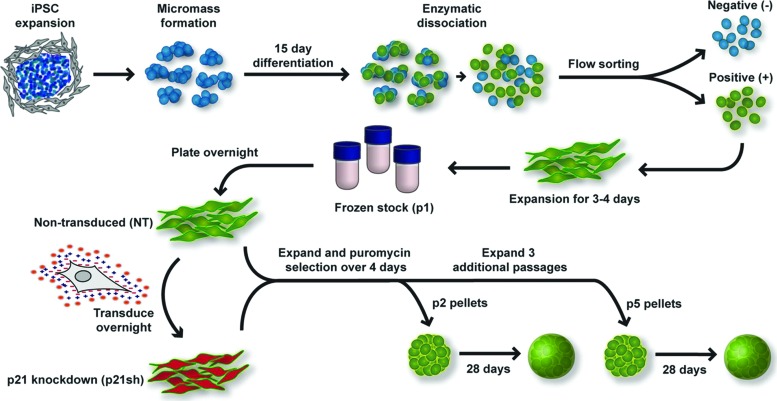
FIG. 1.
Overview of experimental approach. Undifferentiated induced pluripotent stem cells (iPSCs) were expanded on a feeder layer of mouse embryonic fibroblasts, plated in high density micromass cultures for 15 days of differentiation, which included the growth factor bone morphogenetic protein 4 during days 3–5. After differentiation, chondrocyte-like cells expressing green fluorescent protein (GFP) driven by a type II collagen promoter were sorted by flow cytometry. Sorted cells were expanded in primary passage and then stored as frozen stocks for future experiments. Cells were subsequently plated into passage 1 (p1) and transduced the following day with virus encoding short hairpin RNA (shRNA) to reduce the expression of p21 (p21sh) or kept as nontransduced (NT) cells. Both cell groups were expanded and then trypsinized to obtain passage 2 (p2) cells. Cells were either replated for continued expansion or used for quantitative real-time polymerase chain reaction (qRT-PCR) analysis and the formation of chondrogenic pellet cultures. After three additional passages, the same analysis was performed on passage 5 (p5) cells. See Materials and Methods section for additional details and media formulations. Color images available online at www.liebertpub.com/tea