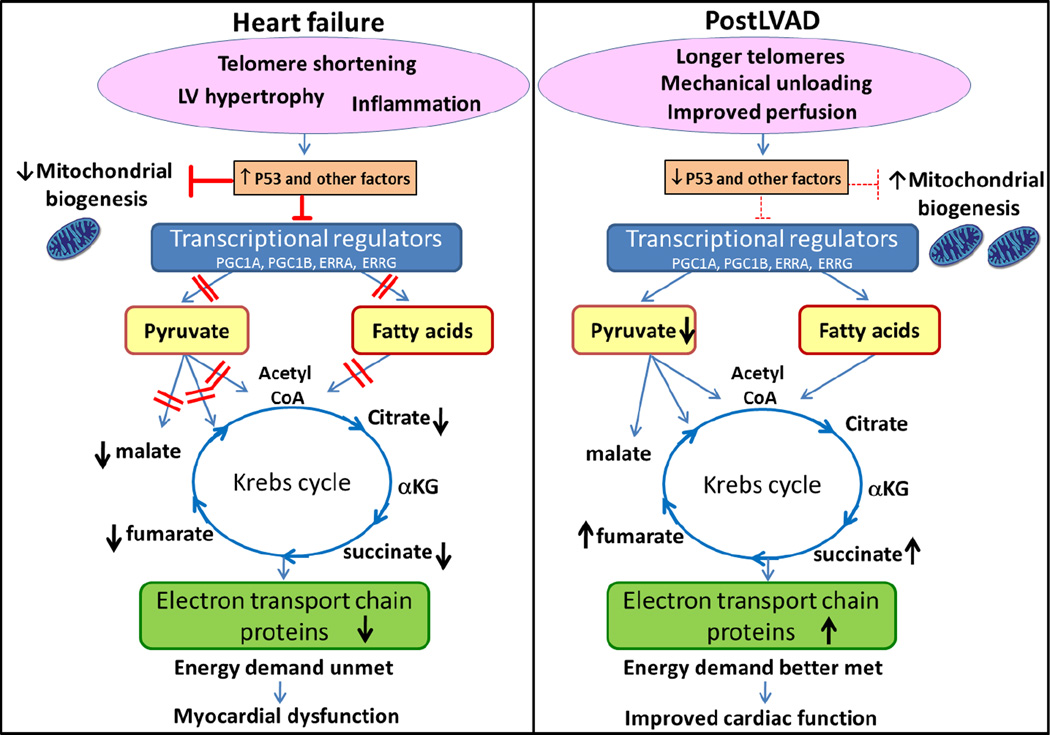
Figure 5.
Mechanical unloading reverses abnormal metabolic profile in failing human heart. In HF, telomere shortening and other deleterious presentations induce one or more regulatory proteins such as P53 which influence the myocardial metabolism. For example, P53 suppresses expression of metabolic transcription factors and inhibits mitochondrial biogenesis in HF. Loss of transcriptional regulation results in impaired β-oxidation and blockage of the various catabolic pathways of pyruvate causing accumulation of pyruvate and reduced Krebs cycle activity. Generalized defects in substrate utilization results in an inability to meet energy demands and ultimately in myocardial dysfunction. LVAD increased length of telomeres which reversed the action of P53, enhancing expression of key transcriptional regulators, and increasing both mitochondrial biogenesis and metabolic enzymes to enhance utilization of pyruvate and fatty acid. These changes post-LVAD enable the heart to better meet energy demands.