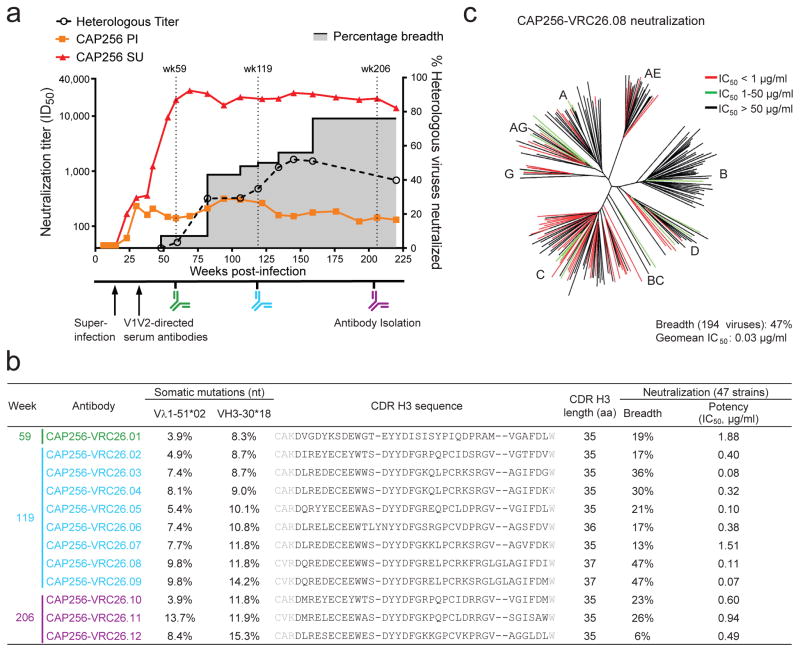
Figure 1. Development of broad neutralization by donor CAP256 and isolation of neutralizing antibodies.
(a) Timing of antibody isolation in relation to plasma neutralization titers against the primary infecting virus (PI), the superinfecting virus (SU), and a panel of 40 heterologous viruses (geometric mean titer shown). Percentage breadth (gray area), % of viruses neutralized with plasma ID50 >45. (b) Genetic characteristics and neutralization breadth and potency of the 12 isolated antibodies. Week of antibody isolation and V-gene mutation rates are indicated. Residues flanking the Kabat-defined CDR H3 sequences are shown in gray. Neutralization was assessed against a panel of 47 heterologous viruses. (c) Breadth and potency of antibody CAP256-VRC26.08 on a panel of 194 Env-pseudoviruses. Dendrogram shows phylogenetic relatedness of Env sequences in the panel.