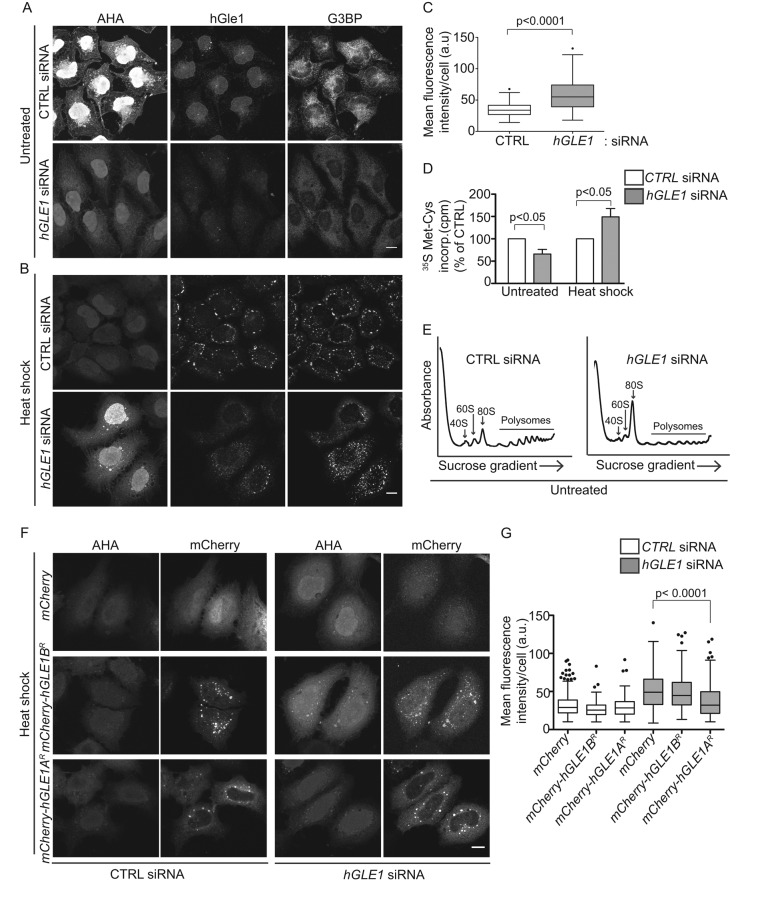
FIGURE 5:
hGle1 modulates SG assembly by regulating translation. (A, B) Nascent protein synthesis is deregulated in hGle1-depleted cells. HeLa cells treated with CTRL and hGLE1 siRNAs were subjected to heat shock at 45°C or left untreated. After 15 min, AHA was added to the incubations, and heat shock treatment was continued for an additional 30 min. Samples were processed by Alexa Fluor-488 alkyne staining followed by immunofluorescence with anti-G3BP and hGle1 antibodies. Scale bar: 10 μm. (C) Quantification of AHA-488 staining. Mean fluorescence intensity of AHA-488 staining in individual cells was calculated in CTRL and hGLE1 siRNA cells using ImageJ. (D) CTRL or hGLE1 siRNA-treated HeLa cells were either heat shocked at 45°C or left untreated followed by metabolic labeling with 100 μCi/ml [35S]methionine/cysteine for 30 min at either 37°C or 45°C. Cells were lysed, and 35S incorporation was measured by liquid scintillation counter. Counts per minutes (cpm) are shown for hGLE1 and CTRL siRNA-treated cells. (E) hGle1-depleted cells have polysome profile defects under normal conditions. CTRL or hGLE1 siRNA cells were lysed, and polysome profiles were generated by subjecting cells to a 7–47% sucrose gradient centrifugation. The 40S, 60S, 80s, and polysome peaks are labeled. (F) Expression of hGle1A but not hGle1B rescues translation defect in hGle1-depleted cells. CTRL or hGLE1 siRNA-treated HeLa cells were transfected with mCherry, mCherry-hGLE1AR, or mCherry-hGLE1BR plasmids, heat shocked, and processed for metabolic labeling using AHA. AHA incorporation was detected with Alexa Fluor-488 alkyne using click chemistry. Scale bar: 10 μm. (G) Quantification of AHA-488 staining in CTRL and hGLE1 siRNA cells expressing the indicated plasmids.