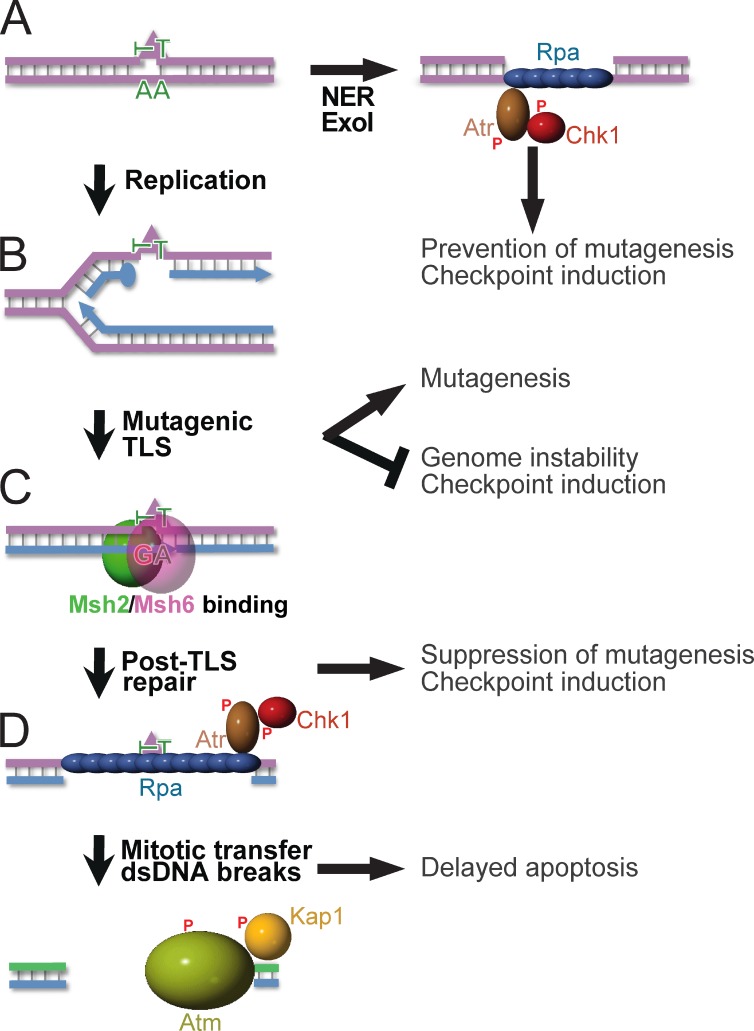
Figure 7.
Orchestration of diverse responses to low-dose UVC by post-TLS repair. P, Phosphate moieties A. NER repairs structurally aberrant DNA lesions (triangle). In nonreplicating cells, Exo1 can lengthen NER-induced excision tracts, provoking Rpa–Atr–Chk1-dependent checkpoint responses (Novarina et al., 2011; Sertic et al., 2011). (B) DNA lesions that escape NER arrest processive DNA polymerases δ or ε, necessitating postreplicative TLS for lesion bypass. By incorporating a nucleotide opposite the lesions, TLS precludes gross-genomic instability and the induction of checkpoints. The frequent incorporation of an incorrect nucleotide at poor or noninstructive lesions causes the inherent mutagenicity of TLS. (C) Msh2/Msh6 specifically recognizes incorrect nucleotides incorporated by TLS. This initiates their excision, which prevents mutagenesis. Persistent excision tracts by post-TLS repair opposite noninstructive lesions underlie the Rpa–Atr–Chk1-mediated intra-S checkpoint. (D) Excision tracts that are transferred to the subsequent cell cycle collapse to dsDNA breaks, reflected by autophosphorylation of Atm and by Atm-dependent phosphorylation of Kap1. We hypothesize that these dsDNA breaks originate from replicative runoff at the gap within the template, and are causative of the delayed Msh2/Msh6-dependent apoptosis.