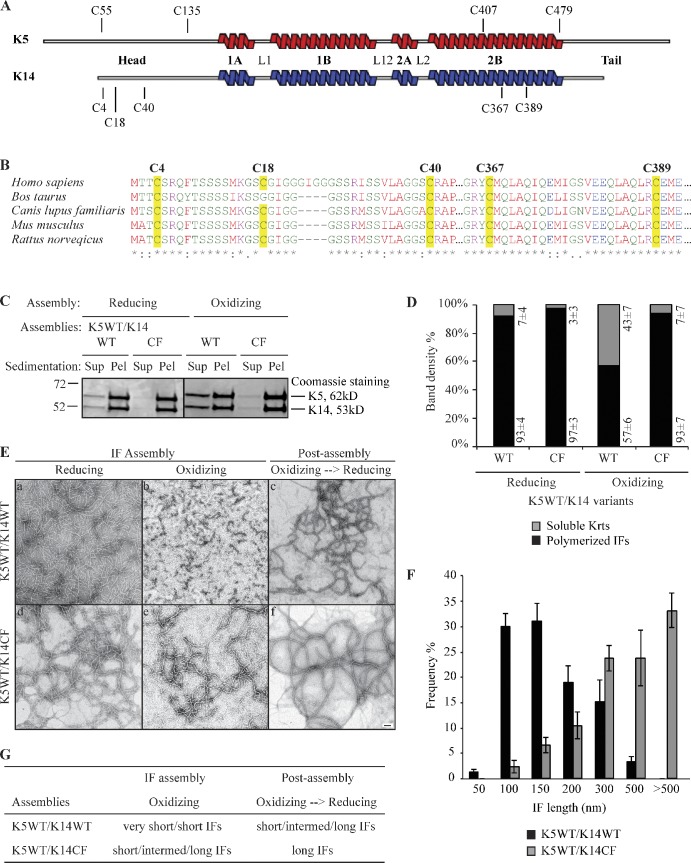
Figure 2.
Limitation of filament elongation via the formation of K14-dependent intermolecular disulfide bonds in vitro. (A) Location of cysteine residues in human K5 and K14 proteins. (B) Alignment of K14 sequence from several mammals: Homo sapiens (NP_000517), Bos Taurus (NP_001160047), Canis lupus familiaris (NP_001240670), Mus musculus (NP_058654), and Rattus norvegicus (NP_001008751). Cysteine residues are highlighted in yellow. (C) High-speed sedimentation of keratin assemblies (150,000 g, 30 min) followed by reducing SDS-PAGE analysis of supernatant (Sup) and pellet (Pel) fractions. K14WT and K14CF have different assembly efficiencies when coassembled with K5WT under oxidizing buffer conditions. The black line indicates that intervening lanes have been spliced out. (D) Quantitation of filament assembly efficiency by densitometry-based analysis of supernatant (S) and pellet (P) fractions as reported in C. Data are presented as the mean ± SD from three independent experiments. (E) Negative staining and transmission electron microscopy showing the structural features of K14WT- and K14CF-containing filaments in an oxidized environment, and upon exposure to a reducing environment at the post-assembly stage. Bar, 100 nm. (F) Measurements of filament length when assembled under oxidizing buffer conditions. Data were obtained from three independent experiments. At least 100 filaments were measured per experiment. Error bars represent SD. (G) Summary of in vitro assembly data for K5/K14WT and K5/K14CF under different buffer conditions. “Very short IFs” are <150 nm, “short IFs” are 200–500 nm, “intermed IFs” are 500–1,000 nm, and “long IFs” are >1 µm.