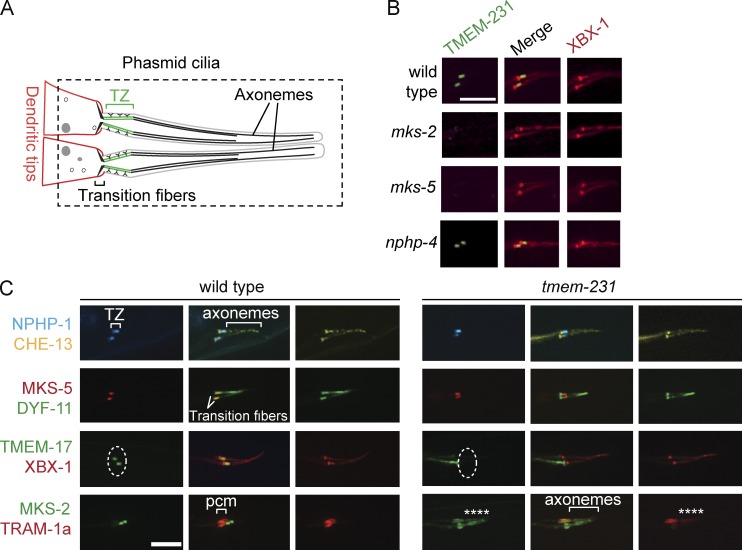
Figure 5.
C. elegans TMEM-231 functionally interacts with other TZ proteins and is required for ciliary gating. (A) Schematic of C. elegans phasmid ciliary structure. The region delineated by the dashed line is depicted in subsequent panels. (B) TMEM-231::GFP (green) is enriched at the TZ of wild-type animals, but is mislocalized in the mks-2 and mks-5 mutants, but not in the nphp-4 mutants. The Dynein 2 component XBX-1::tdTomato (red) marks the basal body transition fibers and axonemes. (C) NPHP-1::CFP (blue) localizes to the TZ of wild-type and tmem-231 mutant animals. Similarly, MKS-5::tdTomato (red) localizes to the TZ of wild-type and tmem-231 mutant animals. CHE-13::YFP (yellow), the C. elegans orthologue of Ift57, and DYF-11::GFP (green), the orthologue of Ift54, mark the transition fibers and axonemes. In contrast, TMEM-17::GFP (green) fails to localize to the TZ (dotted line) in tmem-231 mutants. MKS-2::GFP (green) fails to localize to the TZ in tmem-231 mutants and is instead within the more distal cilia (asterisks). TRAM-1a::tdTomato (red) localizes to the periciliary membrane (pcm) in wild-type animals. However, in tmem-231 mutants, TRAM-1a::tdTomato enters cilia, indicating defects in ciliary gating (asterisks). The dashed ovals delineate the region of the transition zone. Bars, 5 µm.