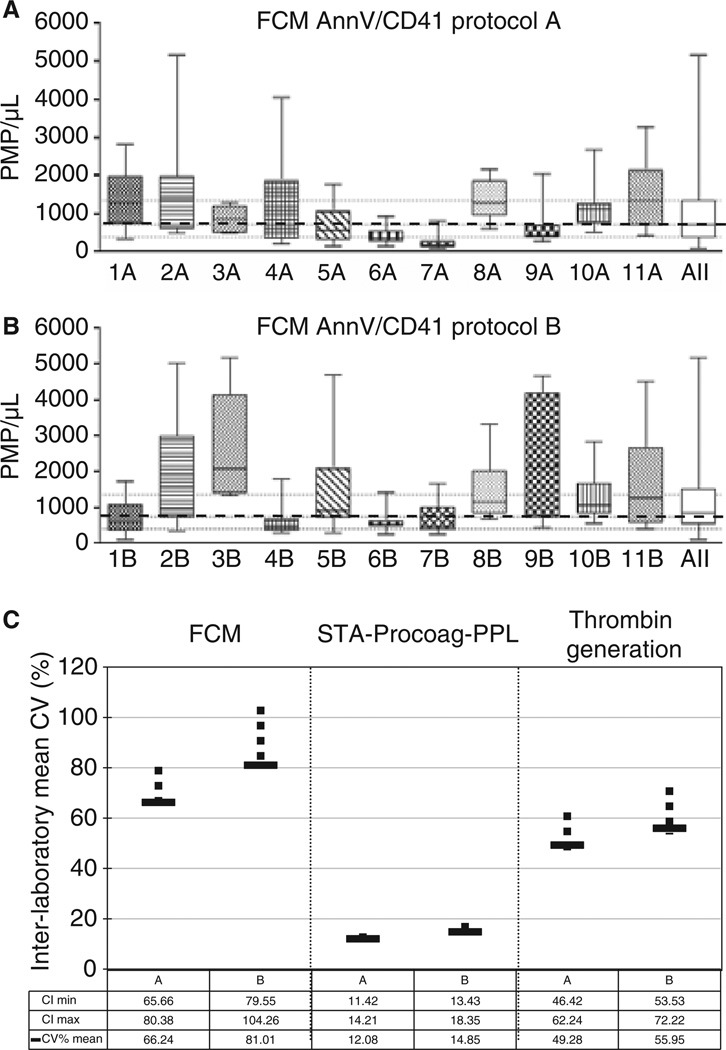
Fig. 1.
Impact of the pre-analytical protocol on the inter-individual and inter-laboratory variability of MP measurements (A & B). Impact of pre-analytics on inter-individual variability of PMP counts by FCM. PMP counts by FCM in normal plasma samples prepared by different laboratories using a common pre-analytical protocol (protocol A, upper graph) or their in house pre-analytical approach (protocol B, lower graph). Median (dashed line) and 25–75% interquartile range (dotted lines) of PMP using protocol A. For example, 1A means laboratory number 1, protocol A. ‘All’ accounts for the combination of data from all the laboratories. (C). Impact of pre-analytics standardization on interlaboratory variability of PMP counts and procoagulant activities. To determine if the inter-laboratory variability was different between protocols for each analyzed parameter, the coefficient of variation was calculated for each laboratory. Then a mean coefficient of variation was derived by averaging all coefficients related on a parameter. Differences between protocols were evaluated used the Bootstrap method and expressed by determination of the confidence intervals. The normal approximations method has been used for computing 95% confidence intervals. CV, coefficient of variation; CI, confidence interval determined by the Boopstrap method (vertical bars).