Abstract
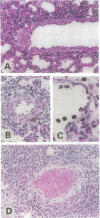
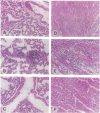
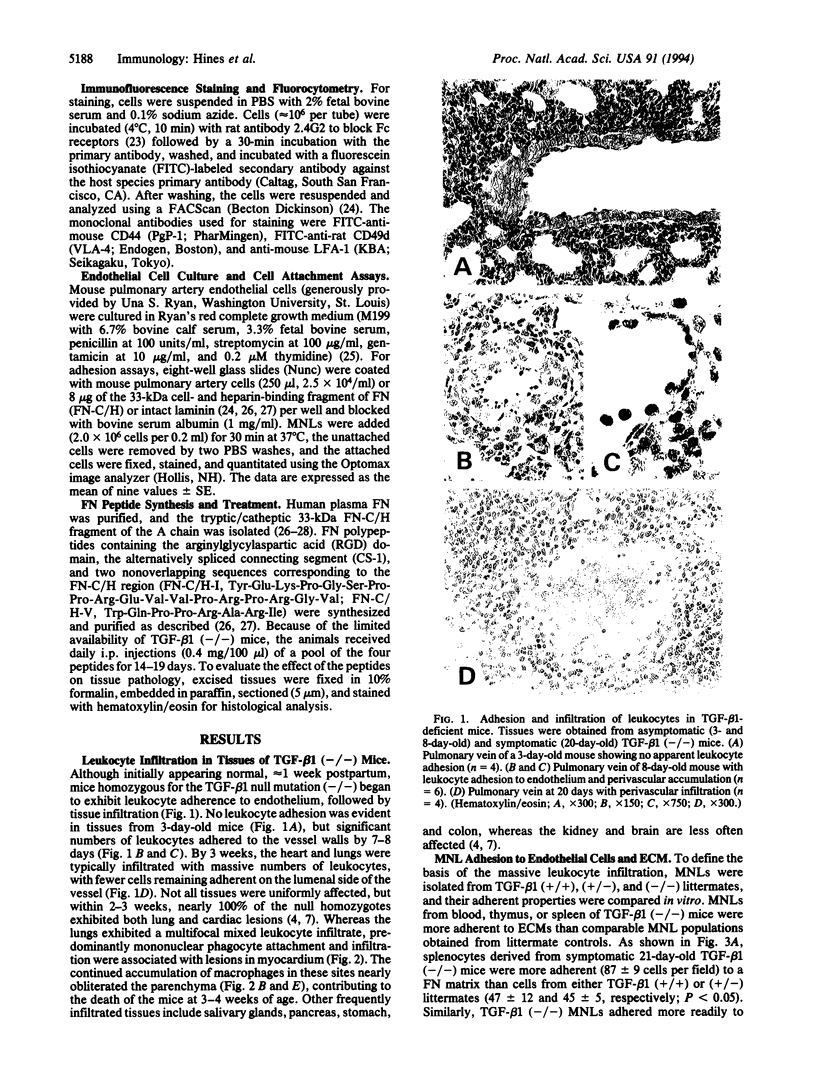
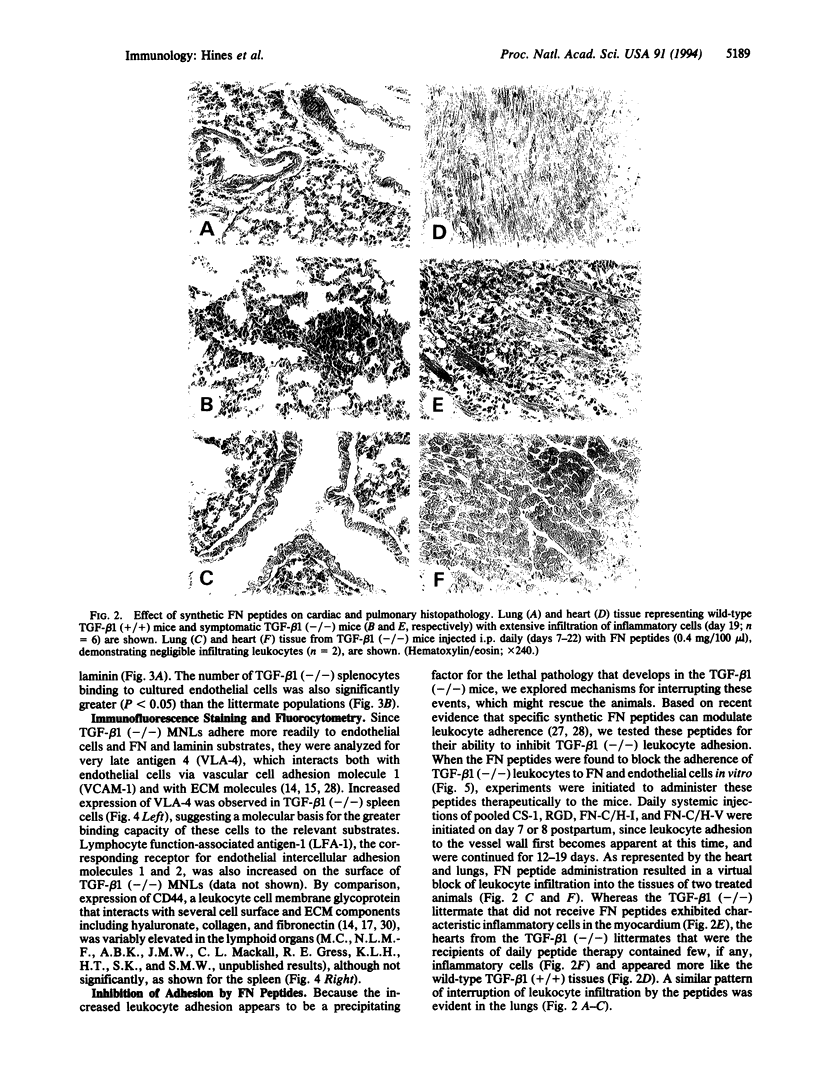
Pronounced mononuclear leukocyte (MNL) infiltration occurs in multiple organs of mice homozygous for a transforming growth factor beta 1 (TGF-beta 1) loss-of-function gene mutation [TGF-beta 1 (-/-)], followed by cachexia and eventually death. Consistent with the increased leukocyte adhesion and tissue infiltration, MNLs isolated from spleen, thymus, and peripheral blood of symptomatic TGF-beta 1 (-/-) mice, as compared to littermate controls, exhibited increased adhesion to extracellular matrix proteins and to endothelial cells in vitro. Incubation of TGF-beta 1 (-/-) MNLs with selected synthetic peptides corresponding to cell- and heparin-binding sequences of fibronectin (FN) significantly attenuated adhesion of these cells not only to FN but also to endothelial cells in vitro. Based on these observations, mice were treated with the FN peptides in an attempt to rescue them from tissue inflammation and cardiopulmonary failure. Daily injections of a combination of four synthetic FN peptides that interact with beta 1-integrins and/or cell surface proteoglycans blocked the massive infiltration of MNLs into the heart and lungs of TGF-beta 1 (-/-) mice. Peptide treatment initiated on day 8, coincident with the first evidence of increased leukocyte-endothelial cell interactions, not only blocked tissue infiltration but also moderated the lethal wasting syndrome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J., FitzPatrick D. R., Gatherer D., Lehnert S. A., Millan F. A. Transforming growth factor betas in mammalian embryogenesis. Prog Growth Factor Res. 1990;2(3):153–168. doi: 10.1016/0955-2235(90)90002-2. [DOI] [PubMed] [Google Scholar]
- Beekhuizen H., van Furth R. Monocyte adherence to human vascular endothelium. J Leukoc Biol. 1993 Oct;54(4):363–378. [PubMed] [Google Scholar]
- Bevilacqua M. P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Brandes M. E., Allen J. B., Ogawa Y., Wahl S. M. Transforming growth factor beta 1 suppresses acute and chronic arthritis in experimental animals. J Clin Invest. 1991 Mar;87(3):1108–1113. doi: 10.1172/JCI115073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser H. M., Yan H. C., Newman P. J., Muller W. A., Buck C. A., Albelda S. M. Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J Biol Chem. 1993 Jul 25;268(21):16037–16046. [PubMed] [Google Scholar]
- Drake S. L., Klein D. J., Mickelson D. J., Oegema T. R., Furcht L. T., McCarthy J. B. Cell surface phosphatidylinositol-anchored heparan sulfate proteoglycan initiates mouse melanoma cell adhesion to a fibronectin-derived, heparin-binding synthetic peptide. J Cell Biol. 1992 Jun;117(6):1331–1341. doi: 10.1083/jcb.117.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. R., Khew-Goodall Y., Vadas M. A. Transforming growth factor-beta inhibits E-selectin expression on human endothelial cells. J Immunol. 1993 May 15;150(10):4494–4503. [PubMed] [Google Scholar]
- Geiser A. G., Letterio J. J., Kulkarni A. B., Karlsson S., Roberts A. B., Sporn M. B. Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9944–9948. doi: 10.1073/pnas.90.21.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine U., Munoz E. F., Flanders K. C., Ellingsworth L. R., Lam H. Y., Thompson N. L., Roberts A. B., Sporn M. B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Iida J., Skubitz A. P., Furcht L. T., Wayner E. A., McCarthy J. B. Coordinate role for cell surface chondroitin sulfate proteoglycan and alpha 4 beta 1 integrin in mediating melanoma cell adhesion to fibronectin. J Cell Biol. 1992 Jul;118(2):431–444. doi: 10.1083/jcb.118.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Mackall C. L., Granger L., Sheard M. A., Cepeda R., Gress R. E. T-cell regeneration after bone marrow transplantation: differential CD45 isoform expression on thymic-derived versus thymic-independent progeny. Blood. 1993 Oct 15;82(8):2585–2594. [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Chelberg M. K., Mickelson D. J., Furcht L. T. Localization and chemical synthesis of fibronectin peptides with melanoma adhesion and heparin binding activities. Biochemistry. 1988 Feb 23;27(4):1380–1388. doi: 10.1021/bi00404a044. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N. L., Wahl S. M. Transforming growth factor beta: a matter of life and death. J Leukoc Biol. 1994 Mar;55(3):401–409. doi: 10.1002/jlb.55.3.401. [DOI] [PubMed] [Google Scholar]
- Norgard-Sumnicht K. E., Varki N. M., Varki A. Calcium-dependent heparin-like ligands for L-selectin in nonlymphoid endothelial cells. Science. 1993 Jul 23;261(5120):480–483. doi: 10.1126/science.7687382. [DOI] [PubMed] [Google Scholar]
- Postigo A. A., Sánchez-Mateos P., Lazarovits A. I., Sánchez-Madrid F., de Landázuri M. O. Alpha 4 beta 7 integrin mediates B cell binding to fibronectin and vascular cell adhesion molecule-1. Expression and function of alpha 4 integrins on human B lymphocytes. J Immunol. 1993 Sep 1;151(5):2471–2483. [PubMed] [Google Scholar]
- Roberts A. B., Roche N. S., Winokur T. S., Burmester J. K., Sporn M. B. Role of transforming growth factor-beta in maintenance of function of cultured neonatal cardiac myocytes. Autocrine action and reversal of damaging effects of interleukin-1. J Clin Invest. 1992 Nov;90(5):2056–2062. doi: 10.1172/JCI116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg C., Postigo A. A., Sikorski E. E., Butcher E. C., Pytela R., Erle D. J. Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J Cell Biol. 1992 Apr;117(1):179–189. doi: 10.1083/jcb.117.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993 Jan 7;361(6407):79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., Weeks B. S., Wong H. L., Klotman P. E. Transforming growth factor beta enhances integrin expression and type IV collagenase secretion in human monocytes. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4577–4581. doi: 10.1073/pnas.90.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M. Transforming growth factor beta (TGF-beta) in inflammation: a cause and a cure. J Clin Immunol. 1992 Mar;12(2):61–74. doi: 10.1007/BF00918135. [DOI] [PubMed] [Google Scholar]
- Woods A., McCarthy J. B., Furcht L. T., Couchman J. R. A synthetic peptide from the COOH-terminal heparin-binding domain of fibronectin promotes focal adhesion formation. Mol Biol Cell. 1993 Jun;4(6):605–613. doi: 10.1091/mbc.4.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]