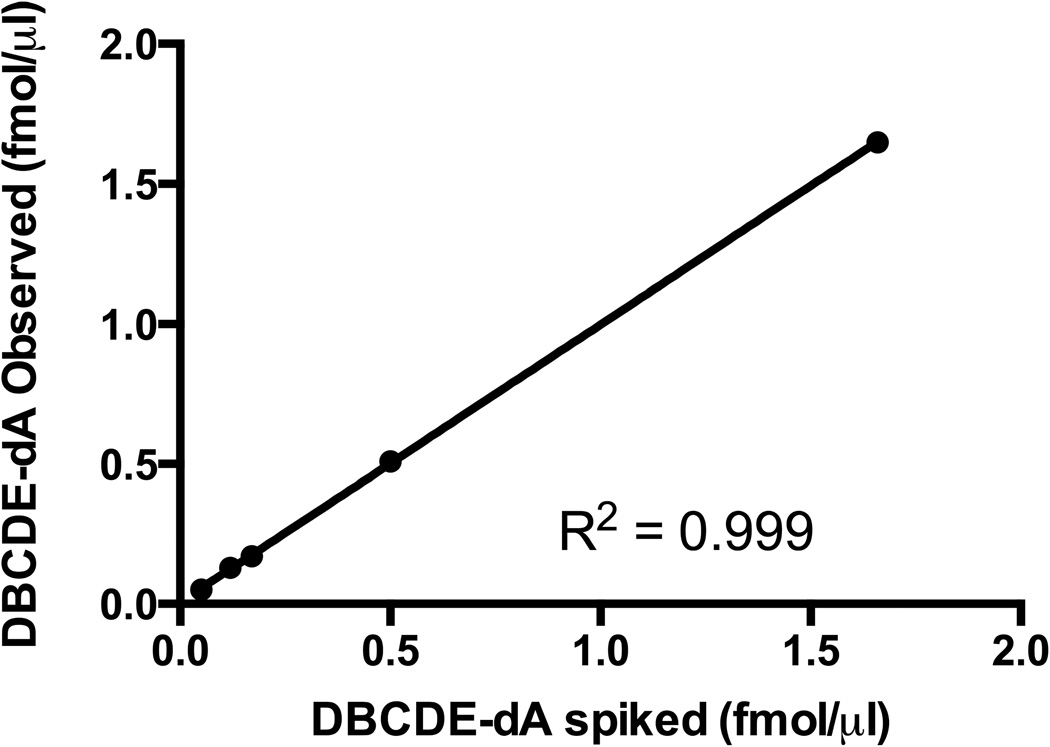
Figure 2. UHPLC-MS/MS method validation.
The stable-isotope dilution UHPLC-MS/MS method was validated using 100 µg spiked control matrix (0.05, 0.12, 0.17, 0.50, 1.66 fmol/µl (±)-anti-cis-DBCDE-dA) and plotting the measured analyte concentrations against the spiked concentrations. Linear regression revealed an R2 of 0.999 with a slope of 0.997 indicating accurate quantitation with the developed protocol. The limit of quantitation (LOQ) was determined to be 0.12 fmol/µg DNA (10 times signal-to-noise ratio).