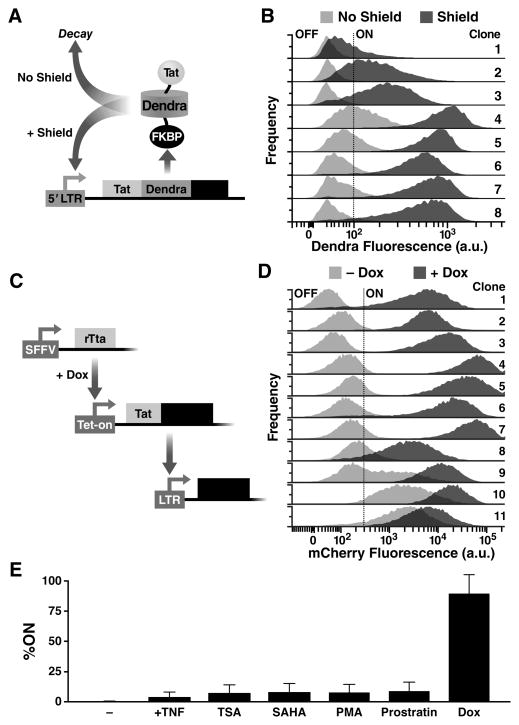
Fig. 4. Synthetic tuning of Tat circuit activity is sufficient to control latent HIV expression in the absence of cellular activation.
(A) Schematic of the minimal LTR-Tat-Dendra-FKBP lentiviral circuit. In the absence of Shield-1, the Tat-Dendra-FKBP fusion protein is rapidly degraded, diminishing positive feedback. When Shield-1 is added, FKBP-mediated proteolysis is blocked, allowing Tat levels to increase and enabling strong Tat positive feedback. (B) Flow cytometry histograms of eight isoclonal populations of Jurkat cells infected with LTR-Tat-Dendra-FKBP in the absence of Shield-1 (light gray histograms) or the presence of 1 μM Shield-1 (dark gray histograms). Gating of the Dendra-positive region (right of black-dashed line) was set relative to naïve, un-transduced Jurkat cells. See also fig. S3 and fig. S4. (C) Schematic of the synthetic system (left) and flow cytometry data of the LTR expression in cells transduced with the synthetic circuit (right). The synthetic circuit is composed of an rTta activator constitutively expressed from an SFFV promoter. In the presence of Dox, rTta protein activates the Tet-On promoter to drive expression of the Tat-Dendra fusion protein. Tat transactivates expression from the HIV-1 LTR promoter and LTR activity is measured by mCherry expression. (D) LTR mCherry expression is shown for 11 representative isoclonal populations in the absence of Dox (light gray histograms) or after Dox addition (dark gray histograms). (E) Flow cytometry analysis of a library containing 33 distinct LTR clonal integration sites subjected to Dox and a panel of standard cell-state modifiers: TNFα, phorbol myristate acetate (PMA), PMA-ionomycin, suberanilohydroxamic acid (SAHA/vorinostat), trichostatin A (TSA), or prostratin. Error bars show standard error.