Abstract
Activation of receptor tyrosine kinase (RTK) signalling pathways is frequently correlated to cancer cell proliferation, angiogenesis and cell survival. Sprouty (SPRY) proteins function as a physiological endogenous inhibitor of RTK signalling pathways, have been shown to be deregulated in most cancer cells. Here, we demonstrated that over-expression of SPRY1 and SPRY2 inhibited B16F10 cell proliferation through G1 phase arrest in vitro, and SPRY2 showed more potent inhibitory effects than SPRY1. In order to tumor-specific delivery of SPRY1/2 in vivo, two strains of attenuated Salmonella typhimurium VNP20009 (VNP-PQE-SPRY1 and VNP-PQE-SPRY2) were constructed to specifically express SPRY1 or SPRY2 under the control of a hypoxia-induced nirB promoter. The efficiency and specificity of the recombinant strains were validated in both bacteria and animal tumor models. SPRY1 and SPRY2 gene could be specifically driven by the nirB promoter under hypoxia, but not normoxia conditions. In addition, the tumor-targeting ability of VNP-PQE-SPRY1 or VNP-PQE-SPRY2 was similar with VNP. VNP-PQE-SPRY2 significantly suppressed melanoma growth in vivo, suggesting that SPRY2 is a more efficient agent for melanoma therapy. Moreover, the antitumor effect of VNP-SPRY2 is mainly mediated through the inhibition of ERK1/2 phosphorylation, which leads to the inhibition of proliferation in melanoma. Taken together, our results indicated that SPRY2 displayed more potent melanoma suppression than SPRY1 both in vitro and in vivo, and the hypoxia-induced tumor-specific gene therapy of SPRY2 delivered by VNP20009 is a promising strategy for melanoma therapy.
Keywords: SPRY1, SPRY2, attenuated Salmonella typhimurium VNP20009, melanoma gene therapy
Introduction
SPRY1 and SPRY2 are members of the SPRY family proteins. They are identified as the endogenous negative regulators of RTK signalling [1,2], which is always aberrantly activated in various tumors such as melanoma [3]. One reason that leads to this continuing activation is the absence of endogenous inhibitors such as SPRY proteins that would otherwise negatively regulate this pathway [4,5]. Also, recent studies have indicated that over-expression of SPRY proteins in tumor cells could lead to the inhibition of tumor growth [6,7]. Thus, SPRY proteins are becoming promising targets for tumor gene therapy. Up to now, the difference in various SPRY proteins is still unknown.
Salmonella typhimurium, which is a kind of facultative anaerobic gram-negative bacteria, can selectively accumulate in solid tumors because of the existence of hypoxic areas within these tumors [8]. Therefore, Salmonella-mediated gene therapy became the focus of cancer research. To reduce the toxicity of the wild strain of Salmonella, many attenuated Salmonella strains were generated. Attenuated Salmonella VNP20009 (purI-/msbB-) is one of the most popular strains used for tumor therapy because of its low toxicity, high tumor targeting, genetically stability, and antibiotic susceptibility [9].Our laboratory and other groups recently reported the use of VNP as a tumor-targeting vector to deliver therapeutic agents to tumor tissues for tumor therapy [10-14].
When Salmonella was internalized by the host cells, it is confined to membrane-bound vacuoles [15]. However, the SPRY proteins have to function in the cytosol. To circumvent this restraint, we made the construct that expressed SPRY1 or SPRY2 fused with the secretion signal peptide of SopE, a natively secreted Salmonella protein that was delivered into the host cell cytosol through type III secretion system [16]. Besides that, as a kind of facultative anaerobic bacteria, Salmonella can also survive and express genes of interest in the normal tissues such as liver and spleen at level, and thus may cause unexpected potential toxicity on these tissues. To further specifically express the genes of interest in tumors and to limit their non-specific expression, we used the anaerobic-inducible nirB promoter to drive the expression of the fused SopE-SPRY1 and SopE-SPRY2 proteins and examined their expression in vitro and in vivo. Moreover, we injected the genetically engineered Salmonella strains into mice carrying melanoma xenografts and found that VNP harboring SPRY2 expressing plasmid showed potent tumor-targeting and tumor-suppressing activity, suggesting a new strategy for melanoma therapy.
Materials and methods
Cell culture
B16F10 melanoma cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 10% FBS, l-glutamine (300 μg/ml), penicillin (100 IU/ml) and streptomycin (100 μg/ml).
Plasmids construction
The full-length coding sequences of mouse SPRY1 and SPRY2 were amplified from the mouse liver cDNA library. For expression in cells, the sequences were cloned into eukaryotic expression vector pRK5-flag, and the resultant vectors were named pRK5-flag-SPRY1 and pRK5-flag-SPRY2. For hypoxia-inducible expression in VNP, the sequences were cloned into the prokaryote expression vector pQE30. As described in our previous study [17], a nirB promoter and the coding sequence of N-terminal amino acids 1-104 of SopE were inserted. The resultant vectors were named pQE30-flag-SPRY1 and pQE30-flag-SPRY2. All the coding sequences of positive clones were confirmed to be correct by DNA sequencing (GenScript Corporation, Nanjing, Jiangsu, China).
Bacteria culture and colony formation assay
S. typhimurium strain VNP (purI-/msbB-) was purchased from American Type Culture Collection (ATCC 202165, Rockville, MD, USA) and grown at 37°C to the mid-logarithmic phase in LB broth. The bacteria were transformed with appropriate vectors using a Gene Pulser apparatus (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions and cultured in aerobic and anaerobic jars. The anaerobic LB medium was prepared by boiling to remove dissolved oxygen. Residual oxygen was driven out further by nitrogen for 15 min in boiling water. Then 0.05% sodium sulfide and 0.05% cysteine were added to maintain the reducing environment in anaerobic jars (Oxid, London, UK). For colony formation assays, the number of CFU was determined as previously described [18].
Proliferation assays
Cells were transfected, and 24 h later, cells were plated in 48-well culture plates at a density of 1 × 104 cells per well in 10% FBS/DMEM. Proliferation was analyzed at different time points using MTT assay. Twenty μl of dimethyl thiazolyl diphenyl was added and the incubation continued for 4-6 h. Medium was removed, and 100 μl dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan by pipetting up and down several times. The absorbance, with a test wavelength of 570 nm and a reference wavelength of 630 nm, was measured. Empty wells (DMSO alone) were used as blanks.
Western blotting analysis
SPRY1 and SPRY2 expressing or empty plasmids were transfected by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Following transfection for 24 h, the cells were collected and homogenized in lysis buffer containing 50 mM Tris-HCl (pH 7.4), 250 mM NaCl, 0.5% Triton X-100, 50 mM NaF, 2 mM EDTA and 1 mM Na3VO4 on ice for 30 min. Anti-Flag, anti-ERK1/2, anti-phospho-ERK1/2 and anti-cyclinD1 antibodies were purchased from Cell Signalling Technology, Inc. (Danvers, MA, USA). Tumor, liver, spleen, lung, kidney tissues were homogenized in lysis buffer on ice for 30 min. Bacterial lysates were prepared from S. typhimurium strain VNP20009 carrying appropriate vectors as described above. Intensity was quantitatively analyzed by Image J software (NIH, Bethesda, MD, USA).
Flow cytometric analysis
Cells were harvested by trypsinization for 48 h after transfection, part of the cells were labeled with FITC-conjugated-annexin V and PI (BD PharMingen, SanDiego, CA, USA) according to the manufacturer’s instructions and analyzed by flow cytometry for apoptosis. The rest cells were fixed with 70% ethanol overnight. The fixed cells were rehydrated in PBS and subjected to PI/RNase staining followed by fluorescence activated cell sorter scan (FACS) analysis (Becton Dickinson, Mountain View, CA, USA). Data were analyzed using FlowJo analysis software (Tree Star, Ashland, OR, USA).
Animal experiments
Six-to-seven-week-old female C57BL/6 mice, purchased from the Laboratory Animal Center, Yangzhou University (Yangzhou, China), were housed in environmentally controlled conditions. The study protocol was approved by local institution review boards and the animal study was carried out in accordance the ethical guidelines for animal use and care established by Nanjing University (Nanjing, China). The C57BL/6 mice were inoculated subcutaneously into the mid-right flank with 5 × 105 B16F10 cells in 0.1 ml PBS. VNP harboring appropriate plasmids were cultured and prepared as described and then injected intraperitoneally with 0.1 ml PBS containing 1 × 105 colony-forming units (cfu) bacteria into the tumor-bearing mice 7 days post-inoculation. Tumor volume was determined using the formula: tumor volume = length × width2 × 0.5.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) and data analysis was performed using Graph Pad Prism version 5.0 (Graph Pad Software, San Diego, CA, USA). Paired Student’s t-test analysis was conducted to assess statistical significance. P < 0.05 was considered to indicate a statistically significant difference.
Results
Overexpression of SPRY1 and SPRY2 inhibit B16F10 melanoma proliferation through G1 phase arrest in vitro
Firstly, the influence of SPRY1 and SPRY2 over-expression on B16F10 melanoma proliferation was examined by MTT assays. Compared with empty vector control, both SPRY1 and SPRY2 induced significant proliferation inhibition in B16F10 cells, and SPRY2 showed a more potent inhibition in comparison with SPRY1 (Figure 1A). To further analyze the mechanisms involving in the SPRY1/2-induced cell proliferation inhibition, the influence of SPRY1 and SPRY2 over-expression on B16F10 apoptosis was investigated. However, there wasn’t any significant difference when SPRY1 or SPRY2 was over-expressed (data not show). Next, the cell cycle phase distribution in SPRY1/2-overexpressed B16F10 cells was examined. Compared with vector control, over-expression of SPRY1 or SPRY2 leaded to an increased percentage of cells in the G1 phase, and a decreased percentage of cells in the G2/M phase (Figure 1C), suggesting that the SPRY1/2-induced cell proliferation inhibition were mainly mediated through G1 phase arrest, not the apoptosis. Western blotting results showed that the activation of ERK1/2 decreased significantly in comparison with SPRY1/2 overexpression and vector control (Figure 1D), suggesting that SPRY1 and SPRY2 also served as negative regulators of MAPK pathway in B16F10 melanoma cells. What’s more, the expression of cyclinD1, a nuclear protein required for cell cycle progression in G1 phase [17], was also significantly decreased, which consolidated the results that SPRY1/2 could induce G1 phase arrest in B16F10 melanoma.
Figure 1.
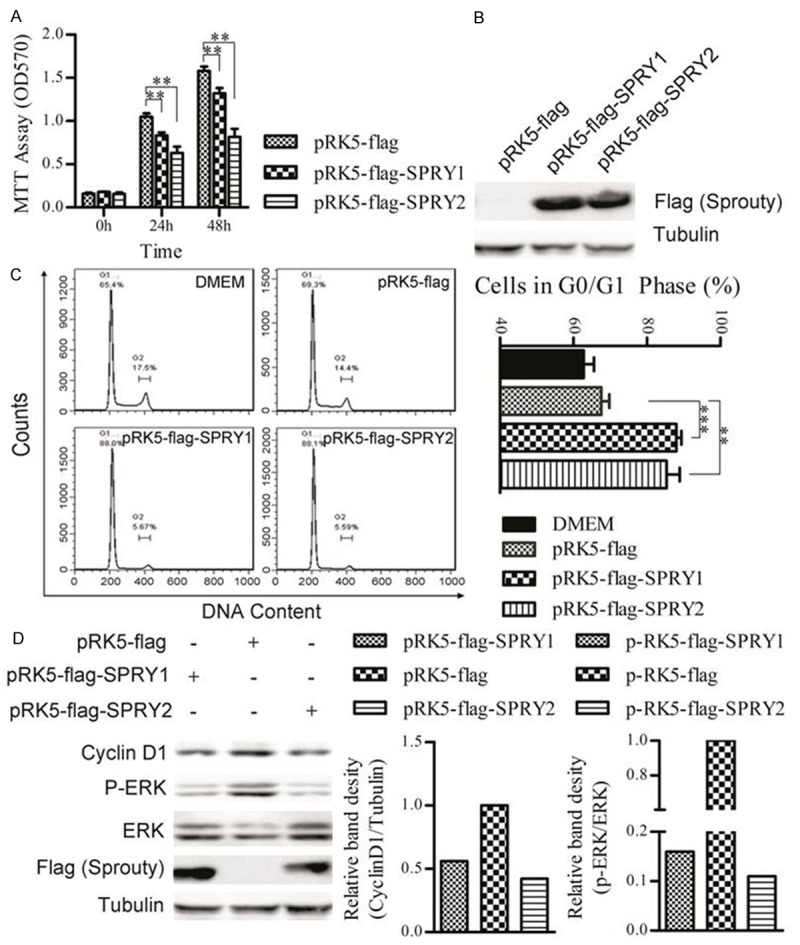
Effects of SPRY1 or SPRY2 overexpression on B16F10 melanoma proliferation in vitro. A. MTT assay of cell proliferation after transfection of SPRY1 or SPRY2 expressing or empty vectors. B. Western blot analysis shows the expression of genes of interest in each group. C. Cell cycle analysis of B16F10 cells that transfected with SPRY1 or SPRY2 expressing or empty vectors. D. Western blot analysis of expression of phospho-ERK1/2, total-ERK1/2 and cyclinD1 after transfection of SPRY1 or SPRY2 expressing or empty plasmids in B16F10 cells. α-tubulin was served as loading control. One representative of three independent experiments is displayed. The extent of ERK activation (p-ERK intensity/total ERK intensity) and relative expression of cyclinD1 were quantified by densitometric analysis.*p < 0.05; **p < 0.01; ***p < 0.001 versus empty plasmids transfection cells. Data are expressed as mean ± SD of three independent experiments.
Construction and characterization of the recombinant salmonella strains for tumor-specific delivery of SPRY1 and SPRY2
The above results demonstrated that an effective proliferation inhibition of B16F10 melanoma cells was achieved by SPRY1 and SPRY2 up-regulation. Next, we try to tumor-specifically deliver SPRY1 and SPRY2 in vivo. Previous studies have proved that the VNP is a promising tumor-target vector for tumor therapy as VNP20009 can specifically accumulate and replicate in the tumors [9]. As shown in Figure 2A, in order to engineer the VNP20009 for tumor-specific delivery of SPRY1 and SPRY2, we used the plasmid pQE30, in which mouse SPRY1 or SPRY2 coding sequence was fused to the sequence of SopE1-104 (the N-terminal 1-104 amino acid residues of SopE which is identified as the secretion signal of type III secretion system). Meanwhile, the coding sequence for Flag tag was inserted between SopE104 and SPRY1/2 genes for the convenience of detection. The expression of the recombinant proteins was driven under the control of a hypoxia-inducible nirB promoter. We transformed the appropriate plasmids into the VNP20009, and the stable recombinant strains were selected by antibiotic-sensitive.
Figure 2.
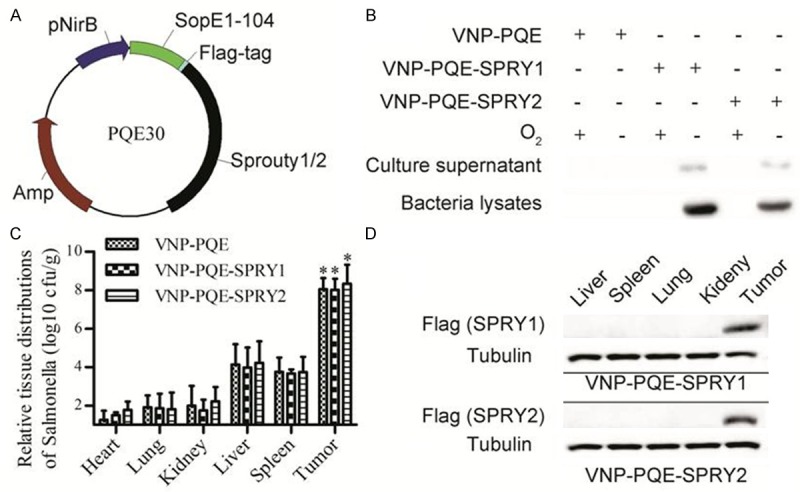
Secretory expression of SPRY1 and SPRY2 by S. typhimurium strain VNP20009. A. Schematic diagram of the construction of vectors for expression of SPRY1 and SPRY2 in VNP20009. B. The indicated stable strains were grew in anaerobic or aerobic jars and their bacteria lysates and culture supernatant were examined for the expression of genes of interest through western blotting as described in the methods. C. The relative tissue distribution of indicated stable strains were detected by colony formation assay. *p < 0.001, tumor compared with the other tissues. Data are expressed as mean ± SD of three animals. D. Detection of the expression of genes of interest in the liver, spleen, lung, kidney, and tumor tissues of the mice bearing melanoma by western blotting. α-tubulin was served as loading control. One representative of three independent experiments is displayed.
Next, to measure SPRY1 and SPRY2 expression, the stable strains were cultured under aerobic and anaerobic conditions respectively. Three days later, the bacteria were harvested by centrifugation and the resulting cell pellet and culture supernatant were tested for the expression of recombinant proteins by western blotting. We found that both SPRY1 and SPRY2 proteins were successfully expressed under anaerobic conditions. Additionally, the recombinant proteins were also detected in the culture supernatant, suggesting the successful secretory expression of these target proteins (Figure 2B). In order to evaluate the effects of tumor-target and the efficiency of SPRY1/2 expression, we injected the recombinant strains VNP-PQE-SPRY1, VNP-PQE-SPRY2 and vector control VNP-PQE into mice bearing B16F10 melanoma xenograft respectively. Three days after treatment with the appropriate strains, mice bearing melanoma were sacrificed and organs were extracted for the analysis of bacteria titer. Colony formation assays revealed that all the strains preferentially grew in tumors when compared with other organs (p < 0.001). There are no significant differences of in vivo distribution between VNP-PQE and VNP-PQE-SPRY1/2 expressing plasmids, suggesting that the expression of exogenous proteins didn’t affect the growth and tumor targeting abilities of VNP20009 (Figure 2C). Next, we examined whether the recombinant proteins were successfully expressed in vivo. Western blotting results showed that both SPRY1 and SPRY2 were only detected in tumors, indicating that the nirB-driven expressions of exogenous proteins were successfully localized in tumor tissues (Figure 2D). Briefly, using this strategy, we successfully make the SPRY1/2 proteins expressed in tumors.
S. typhimurium strain-mediated tumor-targeted delivery of SPRY2 significantly suppresses melanoma xenograft growth in mice
Since the VNP strains could preferentially grow and express the genes of interest in the necrotic areas of the tumors, we further examined the tumor suppressing efficacy of recombinant strains VNP-PQE-SPRY1/2 in vivo. Seven days after the establishment of the melanoma mouse model, mice were treated with appropriate bacteria or PBS control by intraperitoneal injection. Tumor volumes were recorded for the evaluation of the therapeutic efficacy. As shown in Figure 3A, we found that VNP-PQE-SPRY2 significantly inhibited melanoma growth compared with VNP-PQE or PBS group. The tumor doubling time was significantly extended from 3.45 days in vector control group to 4.68 days in VNP-PQE-SPRY2 treated group (Figure 3B). Unexpectedly, although SPRY1 and SPRY2 have similar functions on inhibiting of RTK signalling and proliferation in vitro, VNP-PQE-SPRY1 didn’t show significant antitumor effects compared with the vector control in vivo, suggesting that they might have different functions in vivo. Moreover, immunohistochemical studies revealed that the recombinant proteins were preferentially expressed in the necrotic areas of the tumors (Figure 3C), suggesting that the therapeutic proteins were efficiently expressed under anaerobic conditions. Therefore, the above results reinforce our attempt that Salmonella-mediated expression of therapeutic genes in tumor tissues is an efficient and safety strategy for tumor therapy.
Figure 3.
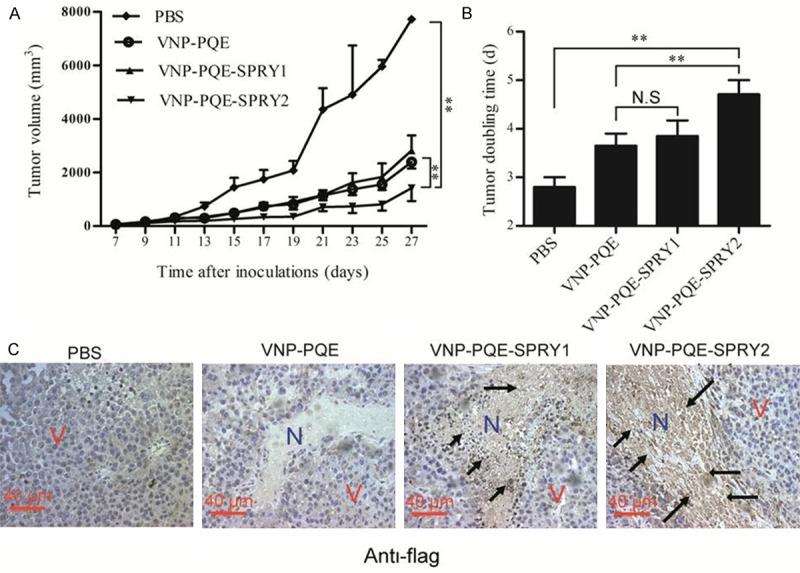
In vivo evaluation of therapeutic efficacy of the recombinant Salmonella. A. Tumor growth curves of groups as indicated. B. Tumor doubling time (time for a tumor to double in volume) of different groups. Data are presented as mean ± SD, *p < 0.05, **p < 0.01 (n = 8 mice), N.S: NS: not significant. C. Detection of the expression of recombinant proteins in the sections of tumor tissues through immune-histochemical studies. N represents necrotic tumor area, and V represents vital tumor cells, black arrows, positive cells.
S. typhimurium strain VNP-PQE-SPRY2 yielded its therapeutic effects through inhibition of ERK1/2 phosphorylation and decreased proliferative capacity in melanoma xenografts
Since SPRY1 and SPRY2 were reported as the inhibitors of the RTK signalling [19], we therefore examined the extent of ERK1/2 activation of tumors when the mice were treated as indicated for 10 days. Tumors tissues were extracted from three mice of every group and analyzed for the expression of phospho-ERK1/2 and total-ERK 1/2 through western blotting (Figure 4A). Our results showed that VNP-PQE-SPRY2 could significantly inhibit the activation of ERK1/2 when compared with the vector group VNP-PQE (Figure 4B). However, VNP-PQE-SPRY1 didn’t show significant inhibition on ERK1/2 phosphorylation, suggesting that SPRY2 is a more efficient agent for melanoma gene therapy. Since the RTK signalling plays a pivotal role in the process of proliferation [20], we next explored the effects of ERK1/2 phosphorylation inhibition on proliferation by using Ki-67 as a marker [21]. Immunohistochemical studies indicated that the Ki-67 index (ratio of the number of positive staining cells to the number of all cells) was significantly deceased when the mice were treated with VNP-PQE-SPRY1 (Figure 4C, 4D), which is consistent with the results of decreased ERK1/2 phosphorylation. Therefore, our findings indicated that VNP-PQE-SPRY1 likely suppressed melanoma growth through proliferation inhibition mediated by ERK pathway.
Figure 4.
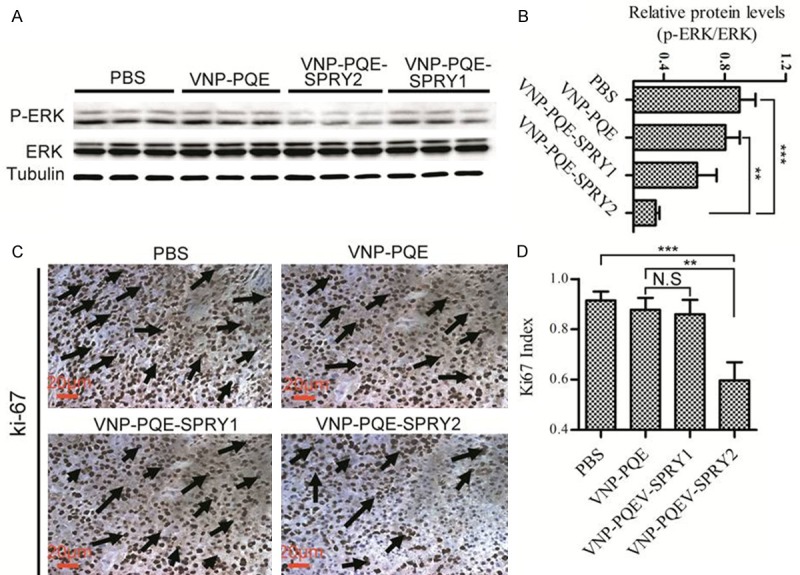
S. typhimurium strain VNP20009 carrying SPRY2 expressing plasmid induced down-regulation of ERK phosphorylation and inhibition of proliferation in tumor tissues of mice bearing melanoma. A. Western blotting analysis of the expression of phospho-ERK and total-ERK in tumor tissues of mice treated with indicated strains. α-tubulin was served as loading control. B. Statistical result of the extent of ERK activation for groups as indicated. C. Immunohistochemical staining of tumor sections of mice treated as indicated with Ki-67 antibody. Black arrows, Ki-67 positive cells. D. Statistical result of Ki-67 index (ratio of the number of positive staining cells to the number of all cells) for groups as indicated. Data are presented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001 (n = 3 mice), N.S: not significant.
Discussion
Salmonella, a kind of facultative anaerobic bacteria, is increasingly investigated in the tumor targeting therapies because of its characteristic of preferential accumulation in the hypoxic and necrotic areas of tumors [22]. Recent studies have showed the efficacy of Salmonella in delivering many kinds of therapeutic agents to the tumor site [23-26]. However, some therapeutic agents could also be detected in the normal tissues, such as liver and spleen, and thus leaded to unintended damages to these tissues. To further reduce the side effects, we used a hypoxia-induced promoter nirB to drive the expression of genes of interest. Western blotting and immunohistochemical studies showed that the recombinant proteins were only expressed under anaerobic conditions both in vitro and in vivo, indicating that this dual targeting strategy is a safety and efficient way to express the genes of interest in tumors.
SPRY proteins are a kind of endogenous inhibitors of RTK signalling, and they exist and play their roles in the cytosol. However, S. typhimurium is confined to membrane bound vacuoles when internalized, which prevents SPRY proteins interacting with other proteins. To solve this problem, we fused the SPRY1 and SPRY2 to the secretion peptide of SopE, an effector protein secreted by Salmonella type III secretion system. Our western blotting results showed that the fused proteins were successfully secreted in vitro. The type III secretion system has been used in the delivery of expressed heterologous antigens to the antigen presenting cells [27,28], but our study first utilized this system to deliver therapeutic proteins into the tumor cells. Moreover, our study also provided an efficient and economic way to over-express intracellular proteins in vivo. It is noteworthy that fusion with secretion peptides might alter the normal structure and functions of therapeutic proteins to some extent, which is one of the limitations of our strategy and needs to be taken into consideration if it was used in other studies.
In this study, we demonstrated that nirB promoter-driven secretory expression of SPRY2 by VNP could markedly inhibit melanoma growth. Further analysis showed that this antitumor effect was mainly mediated through inhibition of proliferation. Salmonella could induce apoptosis and anti-angiogenesis when used as a single antitumor agent [13,29], but it could not inhibit tumor cell proliferation directly, which might be a reason for its limited therapeutic effects. In the present work, we used the S. typhimurium strain VNP20009 to express the RTK signalling inhibitor SPRY1/2 and observed a significantly inhibition on tumor cell proliferation by SPRY2. Thus, the synergistic effect of SPRY2 (proliferation inhibition) and Salmonella (antiangiogenic and proapoptotic effects) results in a more efficient strategy for melanoma therapy.
RTK signalling pathway is presently the hottest drug target for cancer therapy, and dozens of RTK inhibitors have been developed and clinically applied world widely. Since SPRY family members are negative regulators of RTK signalling, we chose two of them, SPRY1 and SPRY2, to examine and compare their ability of inhibiting ERK1/2 activation both in vitro and in vivo. Interestingly, although SPRY1 and SPRY2 belong to the same gene family, SPRY2 exhibited more significant inhibition on ERK1/2 phosphorylation compared with SPRY1, which is consistent with the results of tumor growth curves and doubling times. Thus, the differential inhibition capability of ERK1/2 phosphorylation induced between SPRY1 and SPRY2 might be a reason for their different antitumor efficacy. Additionally, our work showed that SPRY2 is a more potential therapeutic agent than SPRY1 for melanoma gene therapy. SPRY2 is a more ideal potential target, than SPRY1, for melanoma therapy.
In summary, this study demonstrated that S. typhimurium strain VNP20009 harboring SPRY2 expressing plasmid is an effective and promising strategy for the melanoma therapy, and further studies are needed to evaluate its potentiality in the clinical application.
Acknowledgements
The authors are grateful to grants from the Ministry of Science and Technology (2014CB744501, 2012CB967004, 2012AA020809), the Doctoral Station Science Foundation from the Chinese Ministry of Education (20130091130003), the Jiangsu Provincial Nature Science Foundation (BZ2012050 and BE2013630), the State Key Laboratory of Natural and Biomimetic Drugs (K20140201), the Open Project Program of State Key Laboratory of Natural Medicines of China Pharmaceutical University (G140014), the Bureau of Science and Technology of Changzhou (CZ20130011, CE20135013, CZ20120004, CM20122003 and WF201207).
Disclosure of conflict of interest
The authors indicated no potential conflicts of interest.
References
- 1.Casci T, Vinos J, Freeman M. SPRY, an intracellular inhibitor of Ras signaling. Cell. 1999;96:655–665. doi: 10.1016/s0092-8674(00)80576-0. [DOI] [PubMed] [Google Scholar]
- 2.Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. SPRY encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- 3.Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma. Int J Cancer. 2003;104:527–532. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- 4.Fong CW, Chua MS, McKie AB, Ling SH, Mason V, Li R, Yusoff P, Lo TL, Leung HY, So SK, Guy GR. SPRY 2, an inhibitor of mitogen-activated protein kinase signaling, is down-regulated in hepatocellular carcinoma. Cancer Res. 2006;66:2048–2058. doi: 10.1158/0008-5472.CAN-05-1072. [DOI] [PubMed] [Google Scholar]
- 5.Sutterluty H, Mayer CE, Setinek U, Attems J, Ovtcharov S, Mikula M, Mikulits W, Micksche M, Berger W. Down-regulation of SPRY2 in non-small cell lung cancer contributes to tumor malignancy via extracellular signal-regulated kinase pathway-dependent and -independent mechanisms. Mol Cancer Res. 2007;5:509–520. doi: 10.1158/1541-7786.MCR-06-0273. [DOI] [PubMed] [Google Scholar]
- 6.Mekkawy AH, Pourgholami MH, Morris DL. Human SPRY1 suppresses growth, migration, and invasion in human breast cancer cells. Tumour Biol. 2014;35:5037–5048. doi: 10.1007/s13277-014-1665-y. [DOI] [PubMed] [Google Scholar]
- 7.Minowada G, Miller YE. Overexpression of SPRY 2 in mouse lung epithelium inhibits urethane-induced tumorigenesis. Am J Respir Cell Mol Biol. 2009;40:31–37. doi: 10.1165/rcmb.2008-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leschner S, Weiss S. Salmonella-allies in the fight against cancer. J Mol Med (Berl) 2010;88:763–773. doi: 10.1007/s00109-010-0636-z. [DOI] [PubMed] [Google Scholar]
- 9.Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, Bermudes D, Brecher SM, Margitich D, Turnier J, Li Z, Luo X, King I, Zheng LM. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Wei D, Zhuang H, Qiao Y, Tang B, Zhang X, Wei J, Fang S, Chen G, Du P, Huang X, Jiang W, Hu Q, Hua ZC. Proteomic screening of anaerobically regulated promoters from Salmonella and its antitumor applications. Mol Cell Proteomics. 2011;10:M111.009399. doi: 10.1074/mcp.M111.009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Yang B, Cheng X, Qiao Y, Tang B, Chen G, Wei J, Liu X, Cheng W, Du P, Huang X, Jiang W, Hu Q, Hu Y, Li J, Hua ZC. Salmonella-mediated tumor-targeting TRAIL gene therapy significantly suppresses melanoma growth in mouse model. Cancer Sci. 2012;103:325–333. doi: 10.1111/j.1349-7006.2011.02147.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheng X, Zhang X, Cheng W, Chen J, Ma C, Yang B, Hua ZC. Tumor-Specific Delivery of histidine-rich glycoprotein suppresses tumor growth and metastasis by anti-angiogenesis and vessel normalization. Curr Gene Ther. 2014;14:75–78. doi: 10.2174/1566523214666140305223912. [DOI] [PubMed] [Google Scholar]
- 13.Jia LJ, Xu HM, Ma DY, Hu QG, Huang XF, Jiang WH, Li SF, Jia KZ, Huang QL, Hua ZC. Enhanced therapeutic effect by combination of tumor-targeting Salmonella and endostatin in murine melanoma model. Cancer Biol Ther. 2005;4:840–845. doi: 10.4161/cbt.4.8.1891. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Chen J, Tang B, Zhang X, Hua ZC. Systemic administration of attenuated in combination with interleukin-21 for cancer therapy. Mol Clin Oncol. 2013;1:461–465. doi: 10.3892/mco.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finlay BB, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 16.Wood MW, Rosqvist R, Mullan PB, Edwards MH, Galyov EE. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol Microbiol. 1996;22:327–338. doi: 10.1046/j.1365-2958.1996.00116.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Dai Y, Chen J, Wang X, Tang B, Zhu Y, Hua Z. Oral delivery of the Sj23LHD-GST antigen by Salmonella typhimurium type III secretion system protects against Schistosoma japonicum infection in mice. PLoS Neg Trop Dis. 2011;5:e1313. doi: 10.1371/journal.pntd.0001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gahan ME, Webster DE, Wesselingh SL, Strugnell RA. Impact of plasmid stability on oral DNA delivery by Salmonella enteric serovar Typhimurium. Vaccine. 2007;25:1476–1483. doi: 10.1016/j.vaccine.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 19.Hanafusa H, Torii S, Yasunaga T, Nishida E. SPRY1 and SPRY2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol. 2002;4:850–858. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 21.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 22.Lee CH. Engineering bacteria toward tumor targeting for cancer treatment: current state and perspectives. Appl Microbiol Biotechnol. 2012;93:517–523. doi: 10.1007/s00253-011-3695-3. [DOI] [PubMed] [Google Scholar]
- 23.Jiang T, Zhou C, Gu J, Liu Y, Zhao L, Li W, Wang G, Li Y, Cai L. Enhanced therapeutic effect of cisplatin on the prostate cancer in tumor-bearing mice by transfecting the attenuated Salmonella carrying a plasmid co-expressing p53 gene and mdm2 siRNA. Cancer Lett. 2013;337:133–142. doi: 10.1016/j.canlet.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Manuel ER, Blache CA, Paquette R, Kaltcheva TI, Ishizaki H, Ellenhorn JD, Hensel M, Metelitsa L, Diamond DJ. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer Res. 2011;71:4183–4191. doi: 10.1158/0008-5472.CAN-10-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon WS, Chae YS, Hong J, Park YK. Antitumor therapeutic effects of a genetically engineered Salmonella typhimurium harboring TNF-alpha in mice. Appl Microbiol Biotechnol. 2011;89:1807–1819. doi: 10.1007/s00253-010-3006-4. [DOI] [PubMed] [Google Scholar]
- 26.Zeng S, Zhang J, Zhang J, Liu Q, Wang S, Wu S, Peng X, Shao J, Huang W. Suppression of murine melanoma growth by a vaccine of attenuated Salmonella carrying heat shock protein 70 and Herpes simplex virus-thymidine kinase genes. Oncology Rep. 2012;27:798–806. doi: 10.3892/or.2011.1556. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa H, Sato E, Briones G, Chen LM, Matsuo M, Nagata Y, Ritter G, Jäger E, Nomura H, Kondo S, Tawara I, Kato T, Shiku H, Old LJ, Galán JE, Gnjatic S. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panthel K, Meinel KM, Domenech VES, Trulzsch K, Russmann H. Salmonella type III-mediated heterologous antigen delivery: A versatile oral vaccination strategy to induce cellular immunity against infectious agents and tumors. Int J Med Microbiol. 2008;298:99–103. doi: 10.1016/j.ijmm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]


