Abstract
This study was to explore the effect and significance of PI3K signal pathway on mechanism of liver injury in chronic fluorosis. We used 48 Sprague-Dawley rats which were randomly divided into 4 groups according to the body weight, 12 in each group, half of male and female. The control group was fed with the solid feed (the fluorine content was 1.5 mg/kg). The fluorosis animals were fed with the corn containing fluorine content of 17 mg/kg from the endemic fluorosis areas. Blocking agent LY294002 was injected in the blocking group and phosphate buffer solution was injected in the blocking control in the caudal vein with 10 mg/kg once every other day in the one week before the end of the experiment. The animals were drunk by tap water freely. The fluoride contents of urinary and skeletal were determined by the F-ion selective electrode method. The mRNA and protein expressions of PI3K, Akt1 in the liver tissues were determined by real-time polymerase chain reaction, and streptavidin-perosidase and Western blot, respectively. Results showed that fluoride contents of the urine and bone were increased in the fluorosis compared to those in the control. The expression of PI3K and Akt1 mRNA and proteins was significantly increased in fluorosis hepatocytes, and lower than that of the fluorosis in the blocking. The apoptosis and the intracellular calcium concentration were increased. Therefore, we conclude that PI3K-Akt signaling pathway may be one of the signaling pathways in the pathogenesis of liver injury caused by fluorosis.
Keywords: Fluorosis, phosphatidylinositol-3 kinase, serine/threonine protein kinase, PI3K-Akt1 signal pathway
Introduction
Fluorine is an important trace element in the human body, but the long-term intake of fluorine which exceeds the normal physiological needs can cause chronic systemic diseases. The main clinical manifestations are the dental fluorosis and fluorosis of bone and changes in the pathological morphology, function and metabolism of various organs and tissues. Viscera pathological damages in endemic fluorosis are very common. Many pathways are involved in mediating these damages, particularly, phosphatidylinositol-3 kinase (or PI3K) and its downstream molecules serine/threonine protein kinase Akt (or PKB), referred to as PI3K-Akt, are signal pathways that plays an important role in the pathogenesis of fluorosis and skeletal injury [1], but the relationship between fluorosis and liver tissue injury is not very clear. This research intends to study the relationship between the PI3K-Akt pathway, including its related factors, and the pathogenesis of liver injury in fluorosis, investigate whether this signaling pathway changes and how it changes in liver tissue to further clarify the mechanism of hepatic tissue injury in endemic fluorosis in order to provide the experimental basis for the research of pathogenesis of endemic fluorosis and its prevention.
Materials and methods
Grouping and treatments of animals
The experimental animals were provided by the Experimental Animal Center of Medical College of Third Military Medical University. The certificate number of the rats is SCXK (Chongqing) 2007-0005; the body weight is from 80 to 100 g. The control group was fed conventional feed (the fluorine content: 1.5 mg/kg), the fluorosis group was fed with the feed made from the maize (fluorine content: 17 mg/kg) in the endemic fluorosis areas, the fluorine content was 10 mg/kg after feed processing, the blocking group was fed with the same feed as that of the fluorosis group and received the caudal vein injection of blocking agent LY294002, 10 mg/kg, every other day in the one week before the end of the experiment and the control group was fed with the same feed as that of the fluorosis group and received injection of blocking agent diluent PBS in the same way. The rats in all the groups drank freely the tap water. The 24-hour urine of experimental rats was collected nine months after the experiment to determine the urinary fluoride content. SD rats were killed by exsanguinations in the femoral artery and their blood was collected by the centrifugal tube. The serum was stored at the temperature of -80°C in a refrigerator after centrifugation. The liver tissue of rats was taken to be stored at the temperature of -80°C in a refrigerator and in the 10% neutral formaldehyde, respectively.
Chemicals and equipment
The chemicals and equipment were shown in Table 1.
Table 1.
Chemicals and equipment
| Rabbit anti-mouse PI3K, AKT1 and p-PI3K, p-AKT actin polyclonal antibody | Epitmics, GeneTex, Southern California, USA |
|---|---|
| β-Actin reference antibody | Santa Cruz, Gene Company Ltd. Beijing |
| Chemiluminescence assay (ECL) kit | Millipore, Billerica, Massachusetts, USA |
| Chemiluminescence assay (ECL) kit | Millipore, Billerica, Massachusetts, USA |
| Total RNA extraction reagent Trizol | Invitrogen, Carlsbad, California, USA |
| Reverse transcriptase | Thermo, Gene Company Ltd, Beijing, China |
| Real-time quantitative PCR iQ TM SYBR® Green Supermix | Thermo, Gene Company Ltd, Beijing, China |
| Flou3-AM | Dojindo, Gene Company Ltd, Shanghai, China |
| Apoptosis Kit | Promega, madison, wis, USA |
| Total protein extraction kit | Beyotime, Beijing, China |
| Syndecan-4 mRNA primer | Sangon Biotech, Shanghai, China |
| SDS-PAGE gel configuration Kit | Beyotime, Beijing, China |
| BCA protein concentration test Kit | Beijing Dingguo Changsheng Biotechnology, Beijing, China |
| Immunostain SP Kit | Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China |
| DAB Developer Kit | Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China |
| −80°C ultra-low temperature freezer | Forma, California, USA |
| 5810-R refrigerated centrifuge | Eppendorf, Hamburg, Germany |
| Adjustable micro-pipettes | Eppendorf, Hamburg, Germany |
| Olympus CH20 microscope | Leica, Hamburg, Germany |
| Immun-Blot PVDF membrane | Bio-Rad, Hercules, California, USA |
| Autobiochemical machine AU5400 | OLYMPUS, Gene Company Ltd, Beijing, China |
| Chemiluminescent detection | Bio-Rad, Hercules, California, USA |
Dental fluorosis analysis
Dental fluorosis incidences in rats were observed according to the dividing standard of dental fluorosis [2] 6 months after the feeding.
The 24-hour urine of rats were collected and urinary fluoride measured by F-ion selective electrode method, the bone tissue of rats were collected after they were killed, skeletal fluoride detected after cineration in the same method.
By the biuret colorimetry, the following indexes in the serum of rats were determined: total protein (TP), albumin (ALB), globin (G), A/G, total cholesterol (TC), alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT) and lactate dehydrogenase (LDH).
Rat liver samples were fixed in 10% neutral buffered formalin, processed in slice of 3 μm thick at normal temperature, and paraffin-embedded sections were stained with hematoxylin & eosin (H&E). Morphological changes in H&E-stained liver tissue were examined using optical microscopy.
Immunohistochemistry SP method: in accordance with SP-9000 immunohistochemistry kit for operation. The cytoplasm of PI3K and Aktl are stained to show positive staining. PBS was used as blank control instead of the first antibody. Each slice was put under the microscope for random observation at 10 high magnification view, calculating the mean value of positive cells among the Total Cellular Score (TCS). Quantitative protein analysis was made in image analysis system.
Western blot analysis: 100 mg of fresh liver tissue of rats were probed with the homogenate of protein extract for determination of the protein content. Protein was loaded onto a 10% Tris-glycine gel (SDS-PAGE), separated by electrophoresis, and transferred to an Immun-Blot PVDF membrane, incubated overnight at 4°C, probed respectively with PI3K antibody (1:5000), AKT antibody (1:5000) and p-PI3K antibody (1:1000), p-AKT (antibody 1:800), and then they were incubated with the anti-mouse antibody as secondary antibody for 1 hour at room temperature. The membrane was rinsed with enhanced-chemi-luminescence (ECL), and then analyzed by the image analyzer with the beta-actin antibody as internal reference.
100 mg of fresh liver tissue of rats were extracted and the bone tissue RNA assayed in the conventional method for its concentration, purity and integrity; cDNA was reversely transcribed according to the manufacturer’s instructions of the reverse transcription kit. Reverse transcription reactions were assembled as follows: the total volume is 10 μl; 2.0 μl reaction buffer; 0.2 μl upstream primer; 0.2 μl downstream primer ; 0.1 μl ultra pure deoxy-ribonucleoside triphosphate (dNTP); 0.5 μl reverse transcriptase MMLV; 5.0 μl diethypyrocarbonate (DEPC) water; 2.0 μl RNA template; According to NCBI’s Genbank retrieve SD rats housekeeping gene beta-actin sequences of PI3K, Aktl (Table 2), primers of Shanghai General Biological Engineering were designed and synthesized. PCR reactions were assembled as follows: 10 μl SYBR Green I dye; the upstream and downstream primers (10 mol/L), 0.5 μl for each, a total of 1.0 μl; 2.0 μl cDNA template (100 ng); 7.0 μl sterile DEPC water (total reaction volume of 20 μl). 2.0 μl Sterile DEPC water was used as a negative control instead of 1 μl cDNA template. PCR cycling parameters were as follows: 95°C for 30 s; 95°C for 5 s. 61°C for 1 s, 72°C for 30 s (40 cycles), 72°C for 10 min. With SYBR Green I as the fluorescent marker and beta-actin as internal reference, we calculated the ΔΔCt value and indicated the detection results of relative quantification of target gene with 2-ΔΔCt value.
Table 2.
Real time PCR primer sequence
| Gene name | Primer sequence (5’-3’) | Product length (bp) |
|---|---|---|
| PI3K | F AACACAGAAGACCAATACTC | 195 |
| R TTCGCCATCTACCACTAC | ||
| Akt1 | F GTGGCAAGATGTGTATGAG | 195 |
| R CTGGCTGAGTAGGAGAAC | ||
| β-actin | F CACCCGCGAGTACAACCTT | 207 |
| R CCCATACCCACCATCACACC |
Fresh liver tissue were processed by mechanical method and made into cell suspension, adjusting the concentration to 106/ml; 200 μl was taken, and centrifuge the solution in 1000 rpm, 4°C, 5 min, decanted the supernatants, and finally collected the cells; washed the cells two times with 1 ml pre-cooled PBS, centrifuge the solution in 1000 rpm, 4°C, 5 min; adding 2 ml Annexin-V-FITC (20 mg/ml), mixed them gently and evenly, protected from light, placed on the ice for 15 min for Flow Cytometry.
Determination of pathway of intracellular calcium ion of liver cells
Fresh liver tissue were snipped by mechanical method and filtered to be made into cell suspension. After centrifugation for 5 min in 1000 rpm, decanted the supernatant and collected cells, the cell concentration reached 106/ml; and Flou3-AM mother (based) liquor was prepared, mixed it with the cell suspension according to the manufacturer’s instructions of the reagent, for incubation for 60 min, at the temperature of 37°C, and decanted it; after fully washing incubated for 30min, at the temperature of 37°C; and the final result was tested by flow cytometry.
Statistical analysis
SPSS (17.0) (made by Norman H. Nie, C. Hadlai Hull and Dale H. Bent, Stanford University) software was used to analyze the statistical data, statistical significance among groups was determined using analysis of variance (ANOVA), and statistical significance between two groups was assessed by T test. The level of significance was set at P < 0.05.
Results
Incidence of dental fluorosis in rats with chronic fluorosis
The incidence of dental fluorosis in rats is shown in Table 3.
Table 3.
Incidence of dental fluorosis in rats
| Group | Number | The incidence of dental fluorosis | Total | Rate (%) | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Normal | I | II | III | ||||
| Control group | 12 | 12 | 0 | 0 | 0 | 0 | 0 |
| Fluorosis group | 12 | 1 | 3 | 5 | 3 | 11 | 91.67 |
| Blocking group | 12 | 1 | 2 | 4 | 5 | 11 | 91.67 |
| Blocking control group | 12 | 2 | 3 | 2 | 5 | 10 | 83.33 |
Urinary and skeletal fluoride contents
Contents of urinary and skeletal fluoride of rats in the fluorosis group and the blocking control group increased greatly compared to the control group, and contents of urinary and skeletal fluoride of the blocking group was lower than those of the fluorosis group. There were statistically significant differences between each group (P < 0.05) (Table 4).
Table 4.
Urine and skeletal fluoride contents
| Group | Number | Urine fluoride (μg/ml) | Skeletal fluoride (μg/g) |
|---|---|---|---|
| Control | 12 | 1.37 ± 0.51 | 0.66 ± 0.16 |
| Fluorosis | 12 | 7.58 ± 0.93a | 26.81 ± 5.43a |
| Blocking | 12 | 4.31 ± 0.85b | 7.11 ± 1.23b |
| Blocking control | 12 | 6.32 ± 0.91a,c | 23.08 ± 5.65a,c |
Compared with control group, P < 0.05;
Compared with fluorosis group, P < 0.05;
Compared with blocking group, P < 0.05.
Test results of liver function
ALT and AST activity increased while TP and ALB activity decreased in the fluorosis group and the blocking control group compared to the control group.
ALT and AST activity decreased while TP and ALB activity increased in the fluorosis group and the blocking control group compared to the blocking group (Table 5).
Table 5.
Livers alterations in the experimental rats
| Group | ALT (U/L) | AST (U/L) | TP (g/L) | ALB (g/L) |
|---|---|---|---|---|
| Control | 42.85 ± 7.79 | 128.37 ± 16.61 | 75.60 ± 8.54 | 39.05 ± 5.53 |
| Fluorosis | 65.15 ± 12.78a,b | 168.00 ± 69.48a,b | 67.20 ± 4.64a,b | 33.10 ± 2.61a,b |
| Blocking | 47.00 ± 10.05 | 220.07 ± 36.79 | 79.70 ± 5.53 | 41.45 ± 3.09 |
| Blocking control | 54.78 ± 9.47a | 149.25 ± 17.61b | 71.50 ± 5.95b | 36.62 ± 2.92b |
Compared with control group, P < 0.05;
Compared with Blocking group, P < 0.05.
Pathological changes of liver tissue of rats
Observed through light microscopy, compared with the control group (Figure 1A), there appeared edema in the liver cell of rats with fluorosis), small vacuolar degeneration in some cytoplasm of the liver cell, pyknosis in some liver nuclei and mild hyperplasia of connective tissue in hepatic periportal (Figure 1B, 1D).
Figure 1.
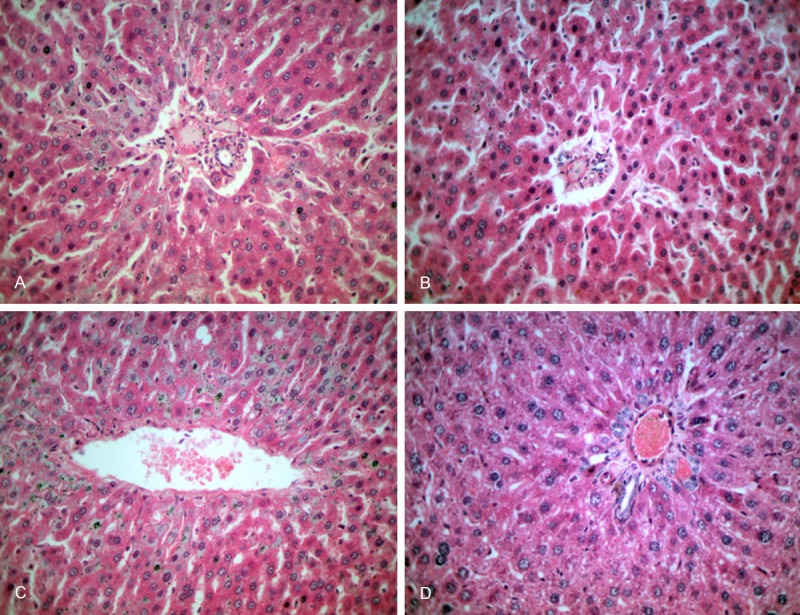
Histological changes in liver tissue were observed with HE (A-D) Control group (A), fluorosis group (B), blocking group (C), blocking control group (D). There appeared edema in the liver cell of rats with fluorosis (B); small vacuolar degeneration in some cytoplasm of the liver cell, pyknosis in some liver nuclei and mild hyperplasia of connective tissue in hepatic periportal (B and D).
Expression of PI3K and AKT protein in rat liver tissue
The immunohistochemical method shows that the PI3K, AKT1 protein positive expression area in liver tissue of rats mainly occurs in the lobular center area the hepatic lobules, the nucleus or cytoplasm looks brownish yellow. PI3K, AKT1 protein expression was negative in the control group; the number of cells of expression in the fluorosis group and the blocking control group increased greatly and showed a deep color, there were less cells of positive expression in the blocking group than in the fluorosis group and the blocking control group. There is obvious difference neither in the comparison between the control group and the blocking group nor in that between the fluorosis group and the blocking control group, but the comparison between the rest groups showed statistically difference (P < 0.05) (Figure 2).
Figure 2.
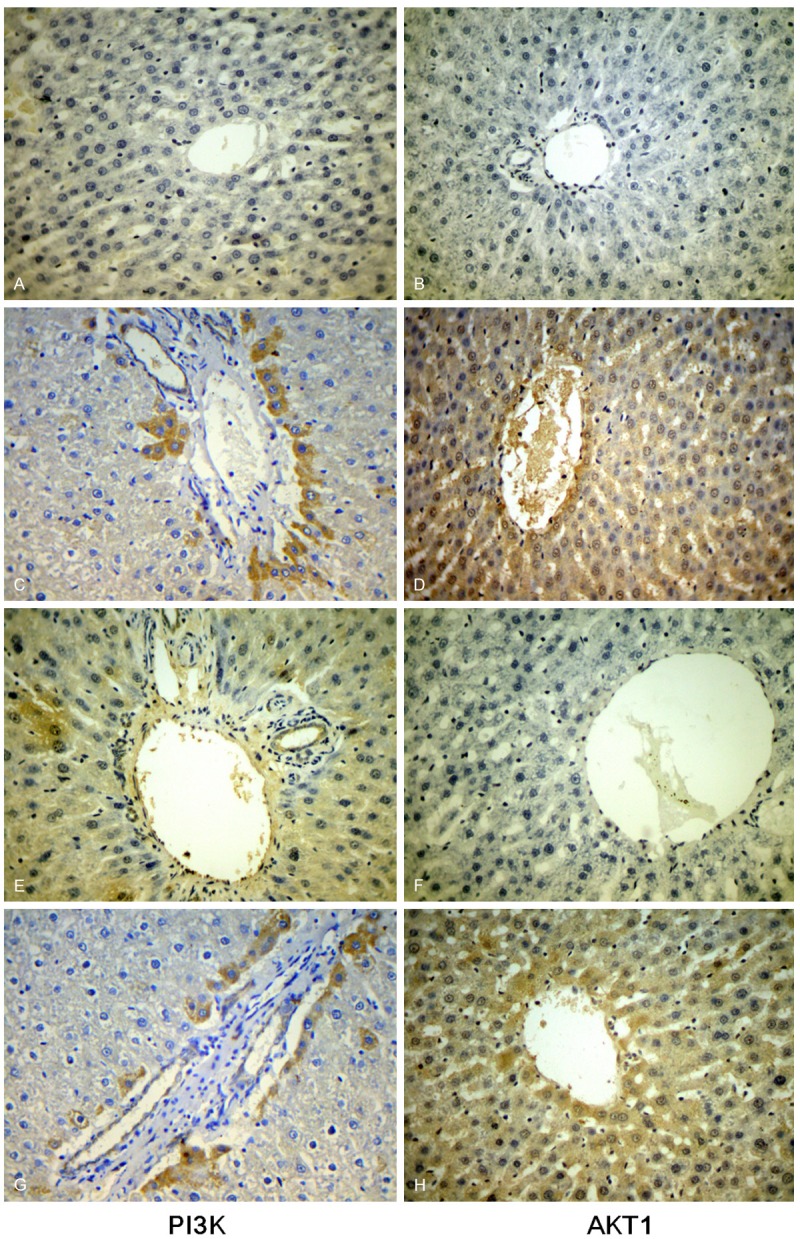
The expression of PI3K and Akt in different groups. Control group (A and B), fluorosis group (C and D), blocking group (E and F), blocking control group (G and H). It shows that PI3K, AKT1 protein expression was negative in the control group while in the fluorosis group and the blocking control group increased greatly and showed a deep color, there were less cells of positive expression in the blocking group than in the fluorosis group and the blocking control group.
Western blot analysis found expressions of PI3K, AKT1, p-PI3K, p-AKT protein in liver tissue of rats of each group. The expression of PI3K and AKT1 protein increased greatly while that of p-PI3K and p-AKT protein decreased (P < 0.05) (Figure 3).
Figure 3.
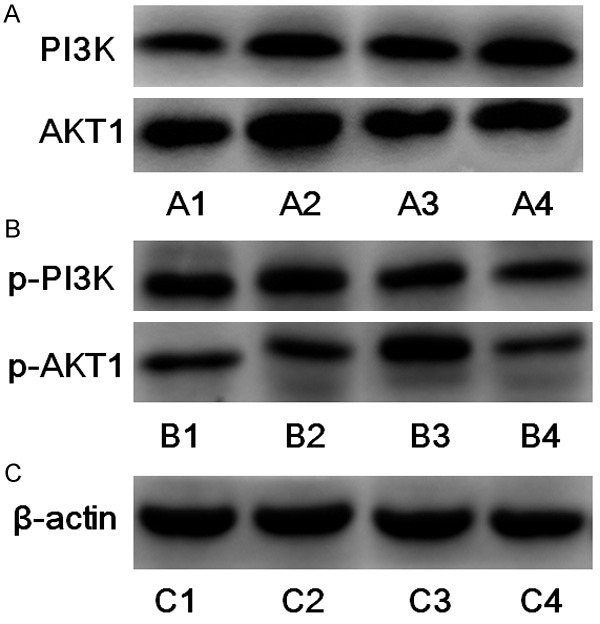
The expression of PI3K, P-PI3K, Akt, p-Akt protein in the hepatocytes of the different groups. The expression of PI3K and AKT1 protein increased greatly (A2 and A4) while that of p-PI3K and p-AKT protein decreased (B2 and B4) (P < 0.05). The expression of β-actin protein in the hepatocytes of the different groups (C1-4).
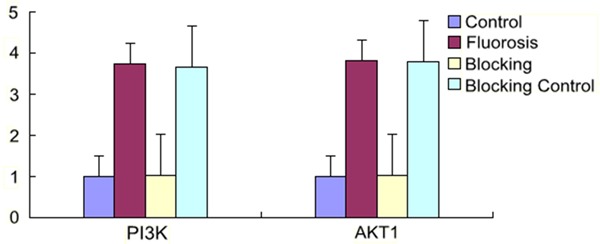
Level of expression of hepatic PI3K, AKT1 mRNA of rats
The expression of the fluorosis group and the blocking control group increased obviously with statistical significance (P < 0.05) compared to the control group and the blocking group. The comparisons both between the fluorosis group and the blocking control group and between the control group and the blocking group showed no statistically significant difference (P > 0.05) (Figure 4).
Figure 4.
Real-time PCR. The expression of the fluorosis group and the blocking control group increased obviously with statistical significance (P<0.05); The comparisons both between the fluorosis group and the blocking control group and between the control group and the blocking group showed no statistically significant difference (P > 0.05).
The apoptosis of liver tissue cell
Flow cytometry analysis showed that there were more hepatic apoptosis cells in the fluorosis group than those in the control group and the blocking group; there were slightly more apoptosis cells in the blocking group than the control group; no obvious difference has been found between the fluorosis group and the blocking control group (Figure 5).
Figure 5.
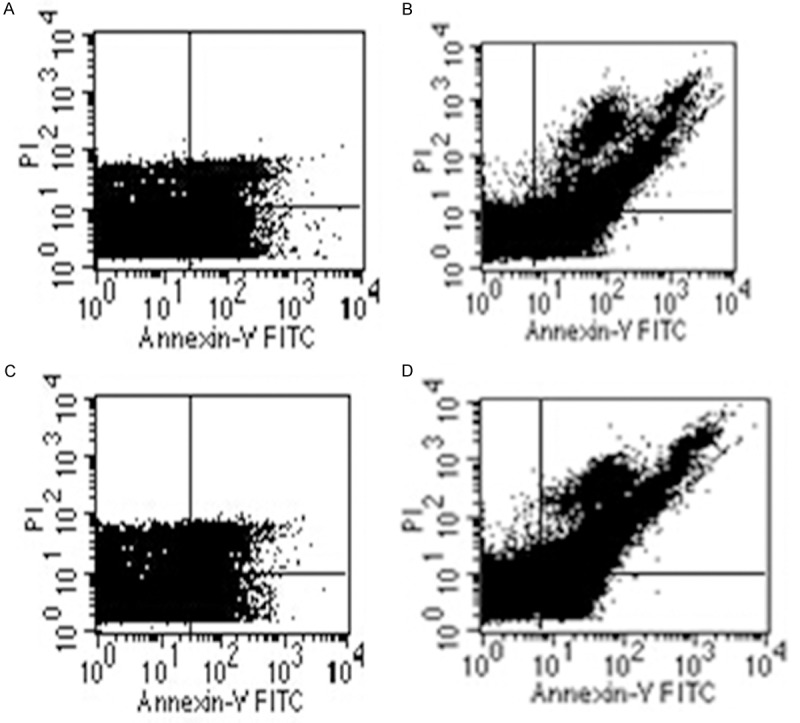
The results apoptosis of rat liver cells (A-D). Control group (A), fluorosis group (B), blocking group (C), blocking control group (D). There were more hepatic apoptosis cells in the fluorosis group than those in the control group and the blocking group; there were slightly more apoptosis cells in the blocking group than the control group; no obvious difference has been found between the fluorosis group and the blocking control group.
Detection of calciumion in the hepatocytes
No obvious difference has been found between the control group and the blocking group; obvious increase of fluorescence intensity has been found in the fluorosis group and the blocking control group, but no obvious difference has been found between them (P < 0.05) (Figure 6).
Figure 6.
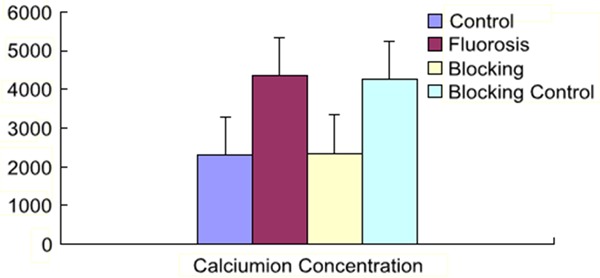
The results of rat liver tissue intracellular calcium. Obvious increase of fluorescence intensity has been found in the fluorosis group and the blocking control group, but no obvious difference has been found between them (P < 0.05).
Discussion
Endemic fluorosis is a global chemical disease. China is one of the countries struck with most serious endemic fluorosis. The clinical manifestations of fluorosis are mainly in teeth and bone damage, but organs and soft tissues such as liver, kidney, nervous system, reproductive system, blood vessels, muscles may also be damaged by this disease [3].
The PI3K family is involved in many signaling pathways and plays a very critical role in stimulating the differentiation, proliferation, survival of cells and angiogenesis [4]. Particularly, the signal pathway composed of IA-type PI3K and its downstream molecule Akt (or PKB) has been greatly concerned by researchers in recent years.
Based on the previous research of our group [5], the rats were provided with the feed made from the maize in the endemic fluorosis areas for 6 months, and detection of dental fluorosis, and skeletal and urinary fluoride contents were made on these rats. Incidences of dental fluorosis in rat’s hepatocytes with chronic fluorosis increased and skeletal and urinary fluoride contents were obviously higher than the control group. This shows that the establishment of the model of animals with chronic fluorosis is successful. And it was found that ALT and AST activity in liver of rats with fluorosis increased while TP activity decreased. This result of research is in agreement with that of other scholars [5-10]. At the same time, it has been found in pathological observation that pathological morphological features of damage appeared in rat hepatocytes with chronic fluorosis.
PI3K is one kind of heterodimer composed of a regulatory subunit (p85) and the catalytic subunit (P110). PI3K forms the receptor tyrosine kinase (RTK) after it is combined with growth factors, and then it forms PIP3 through phosphorylation and exerts its activity by amassing its downstream Akt protein [11].
Our study indicates that PI3K mRNA and its expression level in the hepatocytes with chronic fluorosis rats were increased significantly, compared to the control group; after the use of blocking agent of LY294002, their mRNA and protein expression decreased significantly. And the expression of the phosphorylated PI3K (p-PI3K) in the corresponding mRNA and protein was lower than that of the control group. These results suggest that activation is inhibited when its phosphorylation is blocked, so the protein expression of non-phosphorylated PI3K protein was higher than that of phosphorylated PI3K protein; this also shows that the activation of PI3K-Akt pathway of downstream proteins through pPI3K may be blocked by fluoride.
AKT (PKB), the key factor in the downstream signaling pathways of PI3K, is also an important antiapoptotic regulator [12]. Akt produces an extensive biological effect mainly through phosphorylation [13].
Our study indicated that that AKT1 mRNA and its protein can be promoted increasing by fluoride and be inhibited by the specific inhibitor of PI3K signal pathway in the hepatocytes of fluorosis rats. Reduced expression of p-Akt in hepatocytes shows that Akt phosphorylation is hindered. This will result in weakness of Akt regulation function on cells and cause tissue injury.
AKT activated by PI3K can activate or inhibit downstream target protein Bad, Caspase9, NF-κB, P21, mTOR through its phosphorylation, promote cell growth mediated by insulin and a variety of growth factors (Figure 7). It is an important anti-apoptosis regulating factor which can promote cell survival through a variety of ways [14].
Figure 7.
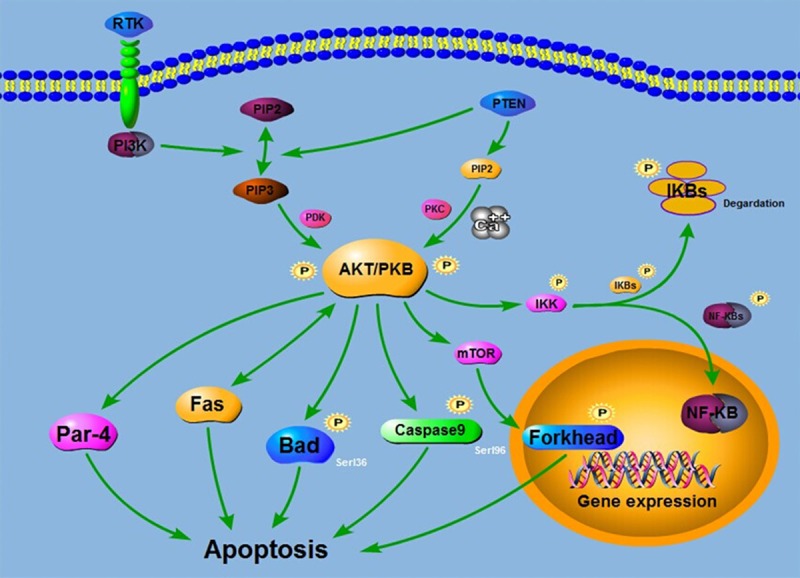
The relationship between various signaling pathways and apoptosis. The diagram, including Bad, Caspase9, NF-κB, Par-4, mTOR and Fas through its phosphorylation signaling pathways, shows the most highly correlated signaling pathways in Apoptosis. These pathways could lead to the apoptosis of cells.
When activated AKT can promote the cell survival, and its major mechanisms are: 1) Direct or indirect activation of functions of Forkhead, CREB and NF-κB; a member of the Forkhead family transfers negatively death promotion signals when it is phosphorylated by AKT while CREB and NF-κB activate the survival pathways. 2) Through phosphorylation of Bad, a Bcl-2 family member, to complete its function to promote cell growth; AKT makes NF-κB phosphorylation, activation of transcription of its functions; when NF-κB is phosphorylated by AKT, its functions of transcription can be activated and expression of members of Bcl-2 family to promote cell survival enhanced [15].
The results indicates that apoptosis be increased in hepatocytes by fluoride in the chronic fluorosis rats. But the inhibitory effect of the blocking agent can reduce the apoptosis of hepatocytes. Taking the previous protein expression into consideration, it may be inferred that the activation of the downstream protein Akt of PI3K will be affected when phosphorylation of PI3K is inhibited. This will weaken functions of the Akt protein to promote cell survival and lead to increased apoptotic cell and to damage the tissues.
Activation of PI3K signaling pathway resulted in the second messenger PIP3 in the plasma membrane, which has a key enzyme PTEN (phosphatase and tensin homology deleted on chromosome ten) for the activation of AKT. PTEN can produce PIP2, which, as a substrate of phospholipase Cβ (PLCβ), produces DAG and IP3 as a second messenger, elevated intracellular calcium levels and active protein kinase C (PKC), so as to exert its biological characteristics.
The results showed that intracellular calcium-ion in rat hepatocytes with chronic fluorosis were significantly increased. It was inferred that due to the obvious increase of PI3K and Akt protein expression, and their phosphorylation blocked in the hepatocytes with chronic fluorosis rat. This promotes the increase of intracellular calcium-ions to play synergistic or complementary roles in the damage of the hepatocytes with fluorosis.
The main biological function of PI3K-Akt signal pathway is to promote cell growth and inhibit the apoptosis of cells [15]. And the main function of the blocking agent LY294002, as one of the most commonly used inhibitors of this pathway, is to inhibit the formation of PIP3, block phosphorylation of PI3K and activation of Akt to produce an inhibition. The phosphorylate of Akt, the key to exert its biological effect, can play a series of protein components and regulate cell apoptosis activity through its effect on numerous target molecules [16-18]. This conclusion is basically consistent with the research results in recent years [19-23]. It is indicates that abnormal changes of the PI3K-Akt signal pathway and intracellular calcium-ion may have relationship with the tissues injury in the fluorosis.
Acknowledgements
All authors thank the participants in the present study for their contributions. This work was supported by The National Natural Science Fund of China (No. 81260419); The Doctoral Fund of Ministry of Education (The Doctoral Superviser) (No. 20125215110001); Special Research and Development Projects of Modernization of Traditional Chinese Medicine of Guizhou Province (No. [2012]5025).
Disclosure of conflict of interest
None.
References
- 1.Zhu H, Yu Y, Deng C, Yang D. The effects of fl uoride on bone tissue of rats with phosphoinositide 3-kinase and protein kinase B1 expression. Journal of Endemic Disease China of Endemic Disease China. 2011;11:261–265. [Google Scholar]
- 2.Zhao L, Yu Y, Deng C. Protein and mRNA expression of Shh, Smo and Gli1 and inhibition by cyclopamine in hepatocytes of rats with chronic fl uorosis. Toxicol Lett. 2014;225:318–324. doi: 10.1016/j.toxlet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Zhao L, Sun Y, Yu G, Sun D. Endemic fl uorosis prevention and control effect evaluation standard of endemic fl uorosis poisoning disease control standard (GB17017-2010) interpretation. Journal of China Health Standard Management. 2011;2:37–40. [Google Scholar]
- 4.Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y. Oxidative stress poisoning in rats in the coal-burning fl uorosis. China Practical Medicine. 2008;3:1–3. [Google Scholar]
- 6.Wu Y, Zhao Q, Zhang Z. Protective effect of selenium on fl uoride induced liver injury in rats. Journal of Environment and Health. 2010;11:955–958. [Google Scholar]
- 7.Zhao Q, Wu Y, Zhang Z. Experimental observation of the protective effect of seleniuminduced renal injury in rats of fl uoride. Journal of Endemic Disease China. 2011;308:137–141. [Google Scholar]
- 8.Sun J, Xu H, Zhang G. The apoptosis of rabbit liver cells and liver function damage infl uorosis. Journal of Endemic Disease China. 2001;11:129–131. [Google Scholar]
- 9.Guo X, Sun G, Sun X. Oxidative stress poisoning liver damage in rats in subchronic fl uorosis. Public Health in China. 2003;12:71–72. [Google Scholar]
- 10.Li H, Yu Y, Cheng Y. Pollution of coal-burning fl uorosis rats Mnsuperoxide dismutase mRNA andprotein levels change. Chinese Journal of Pathology. 2012;41:627–630. [Google Scholar]
- 11.Lin A, Piao H, Zhuang L. FoxO Transcription Factors Promote AKT Ser473 Phosphorylation and Renal Tumor Growth in Response to Pharmacologic Inhibition of the PI3K–AKT Pathway. Cancer Res. 2014;74:1682. doi: 10.1158/0008-5472.CAN-13-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sale EM, Hodgkinson CP, Jones NP, Sale GJ. A new strategy for studying protein kinase B and its three isoforms. Role of protein kinase B in phosphorylating glycogen synthase kinase-3, tuberin, WNKI and ATP citrate lyase. Biochemistry. 2006;45:213–223. doi: 10.1021/bi050287i. [DOI] [PubMed] [Google Scholar]
- 13.Nognchi M, Kinowaki K. P13K- Akt net- work roles in infectious disease. Kansenshogaku Zasshi. 2008;82:161–167. doi: 10.11150/kansenshogakuzasshi1970.82.161. [DOI] [PubMed] [Google Scholar]
- 14.Song G, Ouyang GL, Bao SD. The actinvation of AKT/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sen P, Mukherjee S, Ray D, Raha S. Involvement of the Akt/PKB signaling pathway with disease processes. Mol Cell Biochem. 2003;253:241–246. doi: 10.1023/a:1026020101379. [DOI] [PubMed] [Google Scholar]
- 16.Wong ML, Kaye AH, Hovens CM. Targeting malignant glioma survival to improve clinical outcomes. Clin Neurosci. 2007;14:301–308. doi: 10.1016/j.jocn.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Chen YL, Law PY, Lob HH. Nuclear factor kappaB signaling inopioid functions and receptor gene expression. J Neuroimmune Pharmacol. 2006;1:270–279. doi: 10.1007/s11481-006-9028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Yu Y, Xiao Z. Dan Blue Fairy boron fl uoride treatment capsule colony stimulating factor gene and protein expression level of poisoning rat osteoprotegerin ligand and macrophages on fl uoride. Chinese Journal of Pathology. 2010;39:695–700. [Google Scholar]
- 19.Rao J, Li J, Liu Y, Lu P, Sun X, Sugumaran PK. The key role of PGC-1α in mitochondrial biogenesis and the proliferation of pulmonary arteryvascular smooth muscle cells at an early stage of hypoxic exposure. Mol Cell Biochem. 2012;367:9–18. doi: 10.1007/s11010-012-1313-z. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Wang G, Zhang H, Shen Y, Dai J, Wu L, Zhou J, Jiang Z, Li K. Inability of NS1 protein from an H5N1 infl uenza virus to activate PI3K/Akt signaling pathway correlates to the enhanced virus replication upon PI3K inhibition. Vet Res. 2012;43:36. doi: 10.1186/1297-9716-43-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li HX, Kong FJ, Bai SZ, He W, Xing WJ, Xi YH, Li GW, Guo J, Li HZ, Wu LY, Wang R, Yang GD, Tian Y, Xu CQ. Involvement of calcium-sensing receptor in oxLDL-induced MMP-2 production in vascular smooth muscle cells via PI3K/Akt pathway. Mol Cell Biochem. 2012;362:115–22. doi: 10.1007/s11010-011-1133-6. [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Liu Y, Qiu Z, Liu S, Gao X, Zhu D. Cellular mechanisms and intracellular signaling pathways for the modulation of eNOS in pulmonary arteries by 15-HETE. J Recept Signal Transduct Res. 2012;32:87–95. doi: 10.3109/10799893.2012.660530. [DOI] [PubMed] [Google Scholar]
- 23.Qiu J, Peng Q, Zheng Y, Hu J, Luo X, Teng Y, Jiang T, Yin T, Tang C, Wang G. OxLDL stimulates Id1 nucleocytoplasmic shuttling in endothelial cell angiogenesis via PI3K Pathway. Biochim Biophys Acta. 2012;1821:1361–1369. doi: 10.1016/j.bbalip.2012.07.016. [DOI] [PubMed] [Google Scholar]


