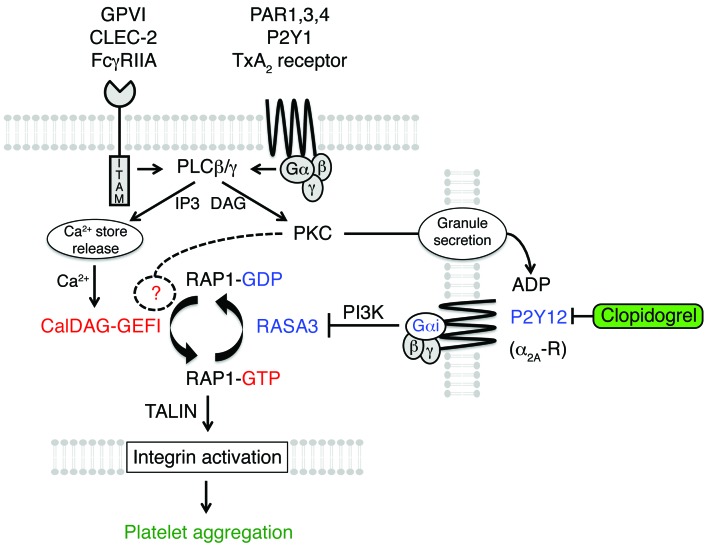
Figure 7. Schematic illustrating the regulation of RAP1 signaling in platelets.
RAP1 activation in platelets is tightly controlled by the antagonistic balance between the calcium-sensitive RAP-GEF, CalDAG-GEFI, and the RAP-GAP, RASA3. In quiescent platelets, RASA3 restrains unwanted CalDAG-GEFI/RAP1 signaling to ensure platelet homeostasis. At sites of vascular injury, platelet stimulation through ITAM-coupled (collagen receptor GPVI, podoplanin receptor CLEC-2, and IgG receptor FcγRIIA) and G protein–coupled (thrombin receptors PAR1/3/4, ADP receptor P2Y1, and TxA2 receptors) receptors leads to the activation of PLCγ2 and PLCβ2/3, respectively. PLCs convert PIP2 to DAG and inositol 1,4,5-trisphosphate (IP3). IP3 mediates Ca2+ store release and a rise in the cytosolic Ca2+ concentration, which triggers rapid CalDAG-GEFI-dependent RAP1 activation. DAG leads to PKC activation, which contributes to nucleotide exchange on RAP1 and the release of platelet granules. CalDAG-GEFI signaling eventually subsides and RASA3 must be inactivated in order to maintain RAP1 in an activated state. RASA3 inactivation depends on PI3 kinase signaling, which in platelets is under the control of Gαi-coupled receptors, such as P2Y12 (ADP) or α2A-R (epinephrine). Both rapid and sustained RAP1 signaling are critical for integrin inside-out activation and the formation of a hemostatic plug. P2Y12 inhibitors prevent the inactivation of RASA3 and thus destabilize the growing thrombus.