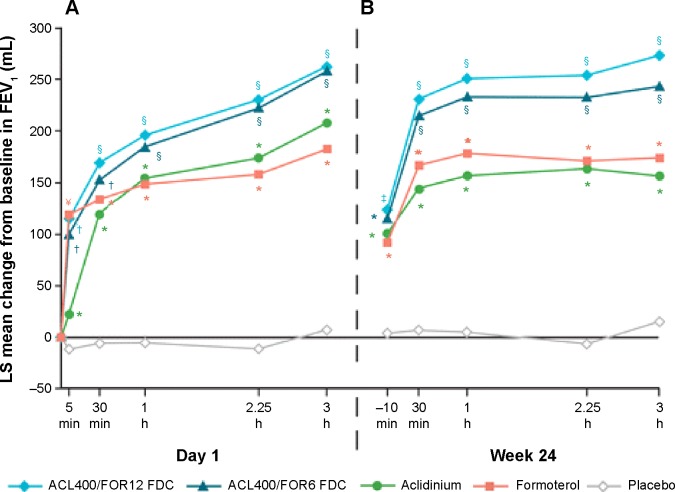
Figure 4.
Mean changes from baseline in FEV1 0–3 hours (A) on day 1 and (B) at week 24.
Notes: Analyses were based on a mixed model for repeated measures. *P<0.05 vs placebo; †P<0.05 vs aclidinium and placebo; §P<0.05 vs aclidinium, formoterol, and placebo; ¥P<0.05 vs aclidinium/formoterol FDC 400/6 μg and placebo. No significant differences between the two FDCs at any time point. Reproduced from D’Urzo AD, Rennard SI, Kerwin EM, Mergel V, Leselbaum AR, Caracta CF; AUGMENT COPD Study Investigators. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15(1):123.44
Abbreviations: ACL, aclidinium; FOR, formoterol; LS, least squares; FEV1, forced expiratory volume in 1 second; FDCs, fixed-dose combinations; ACL400/FOR12 FDC, FDC of aclidinium 400 μg and formoterol 12 μg; ACL400/FOR6 FDC, FDC of aclidinium 400 μg and formoterol 6 μg.