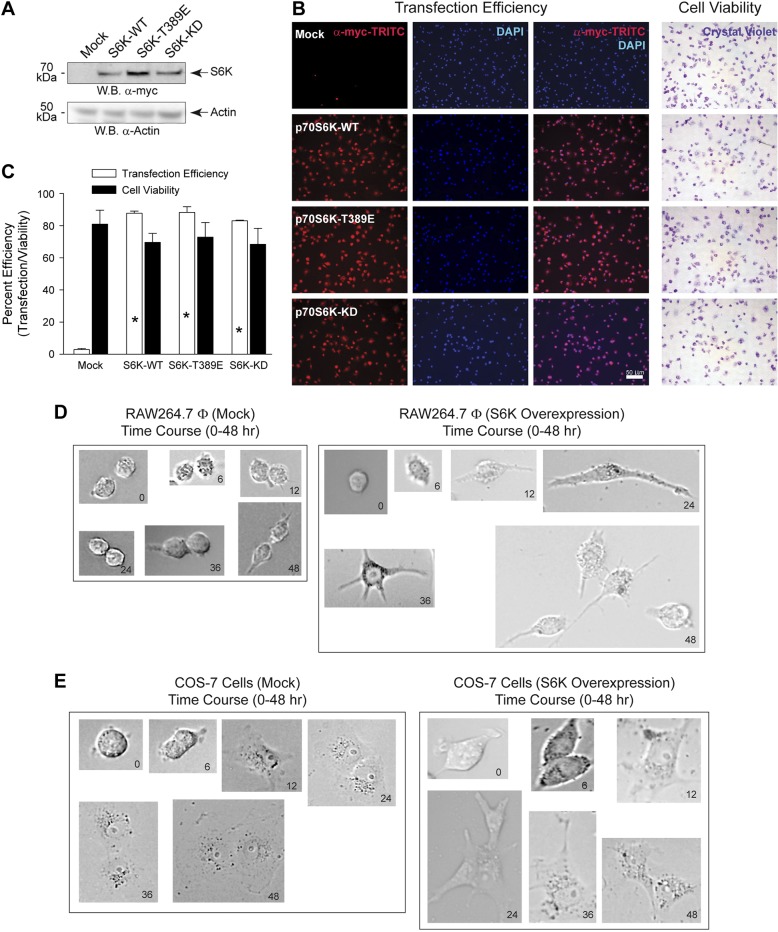
Figure 3.
S6K overexpression does not affect transfection efficiency or cell viability. A–C) RAW 264.7 cells were transfected with 1.5 μg plasmid DNA for 18 h. A) Cell lysates were prepared from S6K transfected cells and used for WB analysis. Effect of overexpression of S6K plasmids is shown. Chemiluminescent detection of the overexpressed 70 kDa myc-tagged S6K protein (upper panel) is denoted on the right by an arrow. Actin is presented as the equal protein loading control (lower panel). Results presented are typical of 3 different assays. B) Cells were incubated with 0.1% crystal violet in 2% EtOH, fixed with paraformaldehyde and then used for immunoblotting using myc-TRITC to detect overexpressed S6K and DAPI to detect nuclei staining. Only viable, live cells will take up crystal violet and result in intense purple staining within the cell. C) Quantification of transfection efficiency and cell viability shown in (B). Data are presented in terms of mean percent transfection efficiency [(red-stained cells only/all cells) ∣ 100%] ± sem or mean percent cell viability [(crystal violet-stained cells only/all cells) ∣ 100%] ± sem. *P < 0.05, statistically significant increase, between samples and controls. D, E) Time lapse change in cell morphology as a result of S6K overexpression. RAW 264.7 (D) or COS-7 (E) cells were transfected with 1.5 μg plasmid DNA for up to 48 hours and bright-field photomicrographs were imaged at 0, 6, 12, 24, 36, and 48 hours post-transfection.