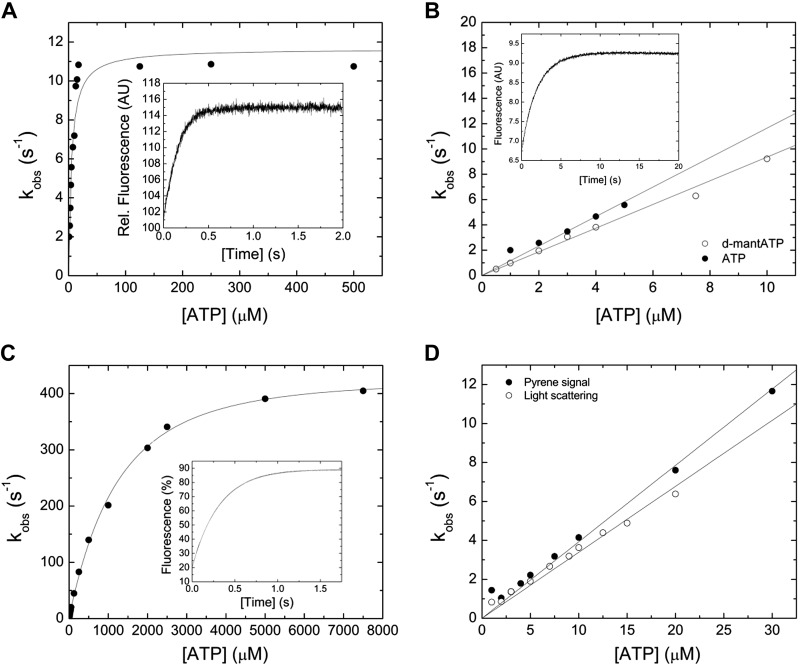
Figure 2.
Interaction between myosin and actomyosin and ATP. A) Increasing [ATP] was mixed under pseudo first-order conditions with 0.25 µM myosin in a stopped-flow spectrophotometer. The dependence of kobs on [ATP] is hyperbolic, yielding the parameter k+3 + k-3 of 11.65 ± 0.56 s−1 at saturation. 1/K1 is observed at [ATP] = 3.73 ± 1.32 µM. The inset shows a representative trace of the transient increase in tryptophan fluorescence after mixing 7.5 µM ATP with 0.25 µM myosin. Single-exponential fit to the data yields an amplitude of 14.2% and a kobs of 6.6 s−1. B) Comparison of the second-order rate constants for ATP and dmantATP binding to myosin at low [nucleotide]. The dependence of kobs on [nucleotide] is linear and results in a K1k+2 of 1.16 ± 0.06 µM−1 s−1 (ATP) and K1k+2 to 0.94 ± 0.02 µM−1 s−1 (d-mantATP). A fluorescence transient of the bimolecular interaction between 0.15 µM myosin and 3 µM d-mantATP is shown in the inset of (B). Single-exponential fit to the data set yields an amplitude of 2.8% and a kobs of 3.06 s−1. C) ATP-induced dissociation of the actomyosin complex. Fluorescence signals were obtained after mixing pyrene-actomyosin under pseudo first-order conditions with increasing [ATP] in a stopped-flow spectrophotometer. Hyperbolic fit to the data yields a k+2 of 482.8 ± 7.85 s−1 at saturating [ATP]. 1/K1 is observed at 1224.37 ± 88.39 µM [ATP]. Inset shows a representative transient of the interaction between 0.5 µM pyrene-actomyosin and 7.5 µM ATP. Single-exponential fit to the data set yields an amplitude of 69% and a kobs of 3.18 s−1. D) Comparison of the kobs upon the ATP-induced dissociation of the actomyosin complex. Monitored is either the postmixing pyrene or the light-scattering signal. Linear fits to the data sets up to 30 µM [ATP] define the second-order rate constants K1k+2 of 0.39 ± 0.01 µM−1 s−1 (pyrene), and K1k+2 of 0.34 ± 0.01 µM−1 s−1 (light-scattering).