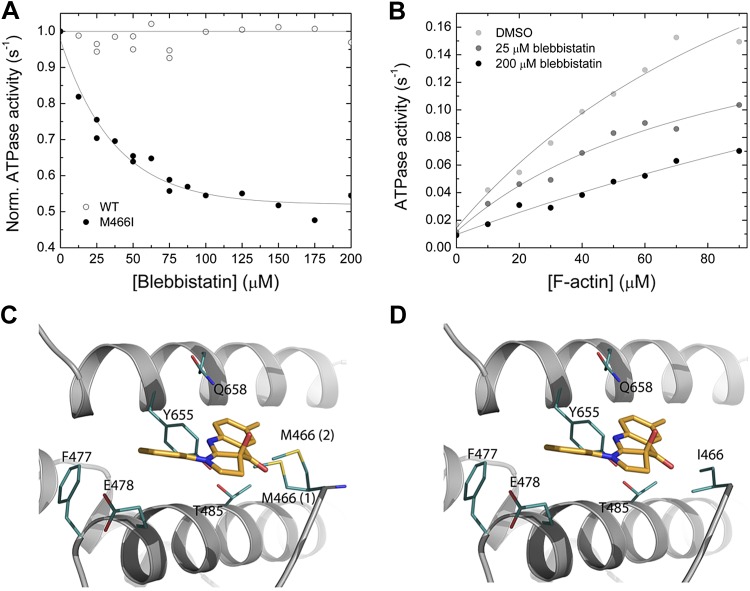
Figure 5.
Interaction between myosin and blebbistatin. A) Normalized (Norm.) actin-activated steady-state ATPase activity of Drosophila nonmuscle myosin-2 (open circles) and M466I (closed circles) as a function of [blebbistatin]. [F-actin] was kept constant at 30 µM, and the data were normalized to the starting value of the ATPase activity in the absence of inhibitor. Single-exponential fit of the data set gives an IC50 of 36.3 ± 4.1 µM for M466I, whereas no inhibition is observed for the native protein [wild-type (WT)]. B) Blebbistatin-mediated decrease of the steady-state actin-activated ATPase activity. Increasing [blebbistatin] leads to a concentration-dependent decrease in the actin-activated ATP turnover. At 90 µM [F-actin], the ATPase activity is reduced >2-fold. For comparison, the uninhibited control was supplemented with 1.8% DMSO to account for the solvent effect. C, D) Close-up views of the blebbistatin binding site in the Drosophila nonmuscle myosin-2 and M466I motor domain homology models in prepower stroke state (ADP·Pi). Key residues are shown as sticks in teal, blebbistatin in mango color. There are 2 major side-chain rotamers [17% (1), and 13% (2)] of Met466 in the active-site loop switch-2 shown for nonmuscle myosin-2 (C). In silico substitution of switch-2 residue methionine 466 by “isoleucine” increases the conformational space available for blebbistatin binding in mutant M466I (D).