Abstract
Objective
To answer frequently asked questions about management of end-stage pneumonia, poor nutritional intake, and dehydration in advanced dementia.
Sources of information
Ovid MEDLINE was searched for relevant articles published until February 2015. No level I studies were identified; most articles provided level III evidence. The symptom management suggestions are partially based on recent participation in a Delphi procedure to develop a guideline for optimal symptom relief for patients with pneumonia and dementia.
Main message
Feeding tubes are not recommended for patients with end-stage dementia. Comfort feeding by hand is preferable. Use of parenteral hydration might be helpful but can also contribute to discomfort at the end of life. Withholding or withdrawing artificial nutrition and hydration is generally not associated with manifestations of discomfort if mouth care is adequate. Because pneumonia usually causes considerable discomfort, clinicians should pay attention to symptom control. Sedation for agitation is often useful in patients with dementia in the terminal phase.
Conclusion
Symptomatic care is an appropriate option for end-stage manifestations of advanced dementia. The proposed symptom management guidelines are based on a literature review and expert consensus.
I introduced Mrs M., an 85-year-old woman with advanced dementia, in part 1 (page 330) of this 2-part review about end-of-life issues in advanced dementia, in which I discussed goals of care, the decision-making process, and how to educate families about therapeutic options.1 In this article, I will focus on symptom management and care issues at the end of life. Recurrent infections and poor nutritional intake are hallmarks of advanced dementia. The appropriate use of antibiotics and artificial nutrition and hydration (ANH) poses many clinical and ethical challenges for the treating physician.
Case description
You and Mrs M.’s daughter have discussed the therapeutic options for Mrs M.’s probable end-stage pneumonia. She agrees that symptomatic care is the most appropriate option at this stage. However, she worries about the pain or discomfort her mother might experience from dehydration and starvation, as well as her pneumonia.
Sources of information
This article is based on a review of literature about end-of-life care in advanced dementia. MEDLINE was searched for articles published until February 2015, containing the following MeSH terms: dementia and palliative care or terminal care; and dementia and pneumonia. A total of 41 articles were retrieved. The search was supplemented with review of related topics in the UpToDate database (www.uptodate.com).
The guidelines for the management of symptoms are also based on my recent participation in a Delphi procedure to develop a guideline for optimal symptom relief for patients with pneumonia and dementia.2
Main message
Poor nutritional intake
Loss of appetite and difficulties with eating and maintaining weight are almost universal and expected complications of progressive dementia, even when appropriately textured food and oral supplements are offered.3,4 Eating problems associated with dementia include difficulty chewing and swallowing, pocketing or spitting, and loss of interest in food. Swallowing problems often lead to aspiration events and pneumonia. Reversible causes of poor intake such as excessive drug sedation, altered mental status due to undetected dehydration, and painful swallowing due to thrush should be looked for and corrected whenever possible.
Feeding tubes
In certain groups, such as patients with severe dysphagia after stroke or for those with amyotrophic lateral sclerosis, a feeding tube can prevent malnutrition and its complications and can prolong life. However, these benefits have not been found in systematic review of the literature among patients with advanced dementia.5,6 This is mostly because there is still a risk of aspiration with a feeding tube. In addition to mortality and morbidity related to the insertion procedure, the tubes cause morbidity from leakage, discomfort, and occasional blocking or displacement that requires an out-patient visit to correct. Furthermore, there might be a risk of needing chemical or physical restraints to prevent the patient from pulling out the tube.7,8
Comfort feeding
Concern about the patient suffering from hunger and thirst is common among families considering tube feeding. Findings of studies in which terminally ill patients still capable of reporting their symptoms were interviewed show that, although it does not provide adequate nutrition, “comfort feeding” is able to eliminate feelings of hunger or thirst. Comfort feeding, or hand feeding, involves giving patients frequent small amounts of food, sips of liquids, or mouth care. This alternative is more aligned with comfort, allows social interaction, and avoids the complications of tube feeding. Cultural and moral standards often impede families from being able to withhold artificial support. Providers must explain to families that careful hand feeding should fill patients’ needs without subjecting them to invasive and nonbeneficial artificial feeding. There is still a risk of aspiration but it is minimized if the feeding is stopped when the patient shows signs of distress.
A “comfort feeding only” order has been proposed. Such an order states what steps are to be taken to ensure the patient’s comfort through an individualized feeding care plan.9 It offers a clear goal-oriented alternative to tube feeding and eliminates the apparent care–no care dichotomy imposed by current orders to forgo ANH. Here is an example of a comfort feeding–only order:
Offer food and liquid as long as it is not distressing.
Feed with the least invasive and potentially most satisfying method.
Provide continuous interaction with the patient (eg, assiduous mouth care, conversation, therapeutic touch).
Artificial hydration
In advanced dementia, the symptoms of dehydration are often nonspecific. It is not rare that dehydration is diagnosed only with marked degrees of volume depletion typically associated with hypernatremia and uremia. Although use of intravenous or subcutaneous clysis might be temporarily useful, many experts think that, in a terminal patient for whom hydration is of questionable value, it might be appropriate to withhold or withdraw this treatment in order to avoid discomfort (prolongation of dying, increased sputum retention).10 However, it must be acknowledged that there are different approaches worldwide that are dependent on the beliefs or attitudes of medical providers and local culture.
Dehydrated patients who are still capable of swallowing should be offered oral fluids and small quantities of food as tolerated. If the effort to eat and drink is too draining or unwelcome, the patient should not be pressured to make this effort. Families who fear that the patient might suffer from thirst should be reassured: the correlation between hydration and thirst status in terminally ill patients is modest. To alleviate symptoms of dry mouth and thirst, mouth care consisting of oral cleaning with swabs and lubrication is recommended every 2 hours.11
Survival and comfort after stopping ANH
A study of predictors of survival among 178 Dutch nursing home patients with dementia who had ANH withdrawn or withheld showed that 59% of patients died within 1 week. Patients who were considered more severely ill, had dyspnea, or experienced apathy were more likely to die within the week. Longest length of survival happened in patients who were still able to take small amounts of fluids.12 Another important finding is that the level of discomfort decreases in the days after the decision is made to forgo ANH. Most patients are probably asleep or comatose shortly before death. Because of the observational nature of the study and the lack of a reference group, conclusions should be drawn carefully. Nevertheless, it seems that forgoing ANH in patients with severe dementia who scarcely or no longer eat or drink is not associated with high levels of discomfort.
Pneumonia
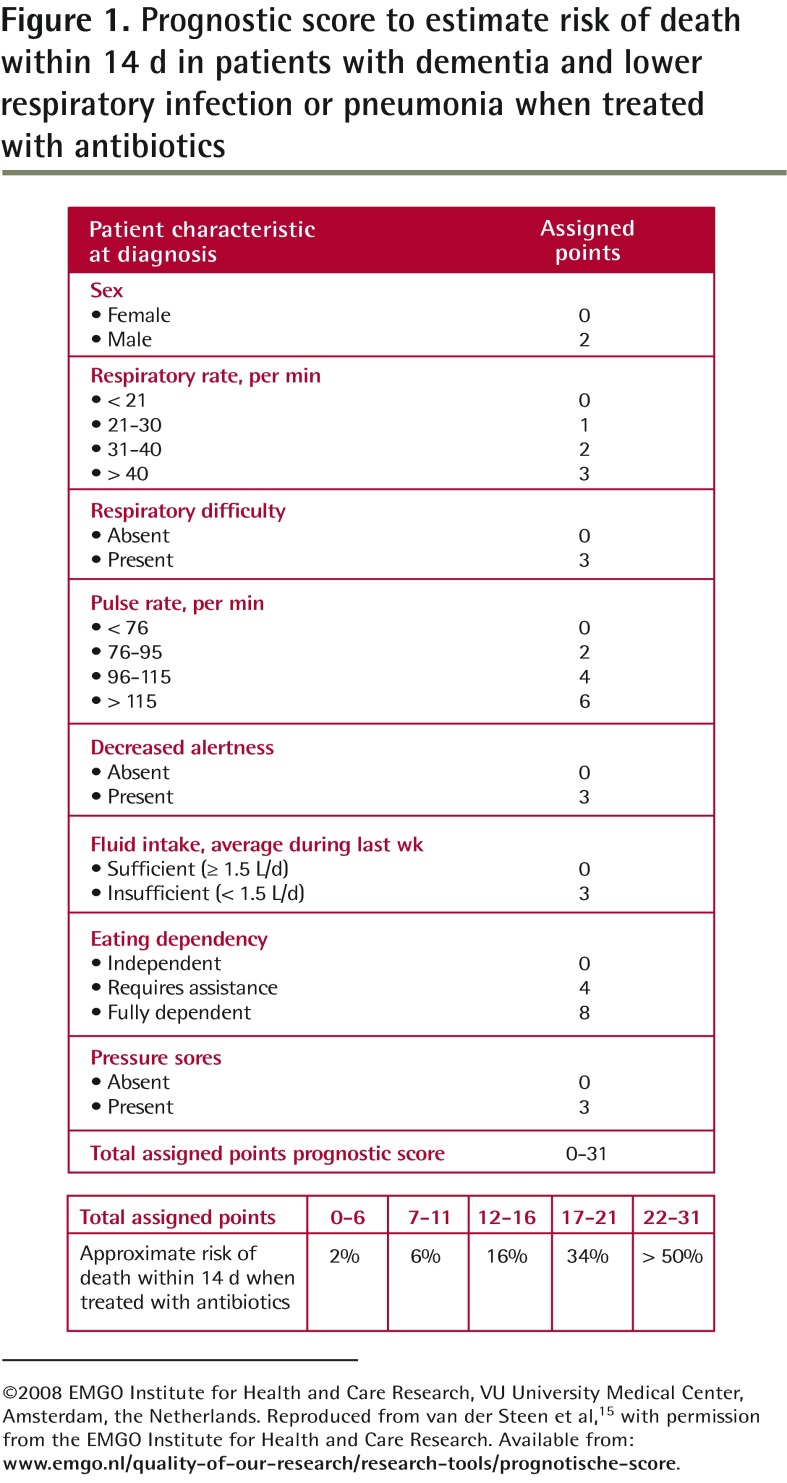
When the patient has very high dependency for activities of daily living, is bedridden, and has dysphagia,13 pneumonia is often the cause of death in the coming weeks or months even if antibiotics are prescribed.13,14 A prognostic score tool (Figure 1) might provide valuable information (both for physicians and family members) for deciding between symptomatic and curative treatment.15 However, decisions should be based primarily on goals of care, taking into account the burden of treatment and the patient’s wishes or best interests according to family and professional caregivers.
Figure 1.
Prognostic score to estimate risk of death within 14 d in patients with dementia and lower respiratory infection or pneumonia when treated with antibiotics
©2008 EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, the Netherlands. Reproduced from van der Steen et al,15 with permission from the EMGO Institute for Health and Care Research. Available from: www.emgo.nl/quality-of-our-research/research-tools/prognotische-score.
Pneumonia is associated with high levels of discomfort, with distressing symptoms occurring more frequently than in patients who die after food and water intake problems.16,17 Antibiotics can be used to increase comfort even when death is imminent. However, this might not be a good idea if the goal of care is symptom control without life prolongation. On the one hand, antibiotics might extend the number of days lived with relative discomfort.18 On the other hand, antibiotics do not always improve comfort, and aggressive approaches (eg, intravenous antibiotics and hospitalization) might decrease comfort. Symptomatic treatment might be more appropriate and includes oxygen, opioids to control pain and dyspnea, anticholinergic drugs such as atropine to reduce respiratory secretions, and benzodiazepines or antipsychotics to reduce fear and agitation (Table 1).2,11,19,20
Table 1.
Guidelines for management of symptoms in end-stage dementia
| SYMPTOM | MANAGEMENT |
|---|---|
| Dyspnea | Identify cause
Use opioids, as they are the most effective treatment
Use 20–40 mg of furosemide (SC or IV) if there is evidence of fluid overload Use oxygen
If dyspnea remains severe, prescribe
Prescribe nonpharmacologic therapy
|
| Terminal rales (“death rattles”) | Reposition the patient, as this is often the best strategy
Start pharmacologic treatment
|
| Pain | Prescribe opioid (oral or SC administration)
|
| Agitation | Consider treatable causes of pain (eg, full bladder, fecaloma, dry mouth) Consider the following:
|
| Myoclonus (frequent side effect of morphine) | Change morphine to hydromorphone and add 0.5 mg of lorazepam every 4 h as needed or regularly |
IM—intramuscular, IV—intravenous, SC—subcutaneous.
Use of scopolamine or other antimuscarinics in this situation is controversial. Some authors believe that this symptom is not burdensome for the comatose patient and that the treatment might not be in the patient’s interest if used only to comfort family.2
Patients who choke on food but have a good capacity to cough might survive aspiration “pneumonitis” caused by irritation of airways by gastric content. Aspiration pneumonitis21 occurs usually after a meal. It presents with acute dyspnea, tachypnea, tachycardia, and fever. It should be managed by raising the head of the bed, clearing the airways gently, providing oxygen therapy, and administering bronchodilators (if there is bronchospasm) and opioids to relieve respiratory distress. Those who can cough effectively often recover within 24 hours. However, many will develop bacterial pneumonia within a few days.
Dyspnea
Most often dyspnea is due to broncho-pneumonia, and a decision to prescribe or to withhold antibiotics has to be made. Opioids are the main symptomatic treatment of dyspnea. Benzodiazepines are helpful if there is anxiety or agitation or severe distress. Sometimes, the acute respiratory distress is due to acute pulmonary edema and should be managed with furosemide and a nitroglycerin patch if the systolic blood pressure is more than 90 mm Hg.
Changes in a dying patient’s breathing pattern might be indicative of considerable neurologic compromise. Periods of apnea or Cheyne-Stokes pattern respirations might develop. Accessory respiratory muscle use might also become prominent. A few last reflex breaths might signal death. Education of family members is often necessary to reduce anxiety and allow them to be reassured about the clinical significance of those manifestations.11
Pain and agitation
Continuous pain in the semiconscious patient might be associated with grimacing and facial tension. The possibility of pain must also be considered when physiologic signs occur, such as transitory tachycardia that might signal distress. However, do not confuse pain with the restlessness, agitation, moaning, and groaning that accompany terminal delirium. If the diagnosis is unclear, a trial of opioids is necessary to judge whether pain is driving the observed behaviour. Some validated pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate22,23 and the Pain Assessment in Advanced Dementia scale,24 used on a regular basis in advanced dementia can be very useful to monitor pain or discomfort in the last weeks of life. Antipsychotics are useful for delirium, but when a patient is terminal and unable to eat or drink anymore, sedation with benzodiazepines might be a better choice. Midazolam or lorazepam given on a regular basis might be indicated for agitation due to fear or possible withdrawal reaction from recently stopped drugs such as benzodiazepines, antipsychotics, and selective serotonin reuptake inhibitors.20,25 More sedation might reduce the need for high doses of opioids, which might bring unwanted side effects such as respiratory depression, nausea, and myoclonus. Furthermore, the advantages of less sedation are not as relevant in a patient with severe dementia as in other palliative care patients who wish to remain in control of their lives for as long as possible.26
Terminal rales
Muscarinic receptor blockers are commonly used to control respiratory secretions. These drugs will minimize gurgling and crackling sounds and can be used prophylactically in the unconscious dying patient; however, there is controversy about their usefulness.2 Some evidence suggests that the earlier the treatment is initiated, the better it works, as larger amounts of secretions in the upper aerodigestive tract are more difficult to eliminate.27 However, premature use in the patient who is still alert might lead to unacceptable drying of oral and pharyngeal mucosa. Ensuring a good body posture (lateral or upright position) might facilitate drainage. In general, avoid suctioning because it might cause gagging or increased mucus production. Families might be disturbed by rattling sounds. Family members should be reassured that these are not burdensome to the comatose patient.
Vital signs, blood tests, and oxygen therapy
During the terminal phase, inappropriate interventions, including blood tests and regular measurement of vital signs, should be discontinued. If the patient appears feverish and has a high temperature, acetaminophen suppositories are often prescribed. Oxygen is useful initially if oxygen saturation is less than 90%, but it might prolong the dying process in patients who are about to die. Oxygen might also be uncomfortable for the patient. After discussion with the family, it can often be stopped when the patient is comatose.28
Case resolution
Mrs M.’s daughter understands that comfort or hand feeding is preferable to tube feeding at this stage of the disease. She contributes to mouth care when she is present. She also understands how opioids, sedatives, etc, will be used to keep her mother as symptom free as possible. Mrs M. initially appears quite uncomfortable and is treated with regular small doses of opioids, scopolamine, and lorazepam. She is now sleeping and comfortable. The family has time to gather and offer her daughter some relief. Mrs M. dies peacefully 4 days later.
Conclusion
The appropriate use of ANH and antibiotics poses many clinical and ethical challenges for the treating physician. Providing a comfort feeding–only order offers a clear goal-oriented alternative to tube feeding. Withholding or withdrawing ANH is generally not associated with manifestations of discomfort if mouth care is adequate. Because pneumonia usually causes considerable discomfort, clinicians should pay attention to symptom control. Palliative “symptomatic care only” is an appropriate option for end-stage manifestations of advanced dementia.
Acknowledgments
Dr Arcand received a Visiting Scholar Fellowship from the University of British Columbia arm of the Canadian Dementia Knowledge Translation Network (Canadian Institutes of Health Research DIP #87302) and support from the National Core for Neuroethics at the University of British Columbia.
EDITOR’S KEY POINTS
For patients with end-stage dementia, comfort feeding by hand is preferable to tube feeding, as it is aligned with comfort, allows social interaction, and avoids complications of tube feeding. A “comfort feeding only” order can provide steps to ensure a patient’s comfort through an individualized feeding care plan.
Patients with advanced dementia might find the effort to eat and drink draining or unwelcome and should not be pressured to make this effort. Symptoms of dry mouth and thirst can be alleviated with mouth care consisting of oral cleaning with swabs and lubrication.
Antibiotics are sometimes prescribed to patients with dementia and end-stage pneumonia to increase comfort even when death is imminent. Withholding antibiotics might be more appropriate if the goal of care is symptom control without life prolongation.
Footnotes
This article is eligible for Mainpro-M1 credits. To earn credits, go to www.cfp.ca and click on the Mainpro link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro d’avril 2015 à la page e183.
Competing interests
None declared
References
- 1.Arcand M. End-of-life issues in advanced dementia. Part 1: goals of care, decision-making process, and family education. Can Fam Physician. 2015;61:330–4. (Eng), e178–82 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Maaden T, van der Steen JT, de Vet HC, Achterberg WP, Boersma F, Schols JM, et al. Development of a practice guideline for optimal symptom relief for patients with pneumonia and dementia in nursing homes using a Delphi study. Int J Geriatr Psychiatry. 2014 Jul 7; doi: 10.1002/gps.4167. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Gillick MR, Yurkofsky M. Medical care in skilled nursing facilities (SNFs) in the United States. Waltham, MA: UpToDate; 2015. [Google Scholar]
- 4.Winzelberg GS, Hanson LC. Palliative care: nursing home. Waltham, MA: UpToDate; 2013. [Google Scholar]
- 5.Pinderhughes ST, Morrison RS. Evidence-based approach to management of fever in patients with end-stage dementia. J Palliat Med. 2003;6(3):351–4. doi: 10.1089/109662103322144664. [DOI] [PubMed] [Google Scholar]
- 6.Finucane TE, Christmas C, Travis K. Tube feeding in patients with advanced dementia: a review of the evidence. JAMA. 1999;282(14):1365–70. doi: 10.1001/jama.282.14.1365. [DOI] [PubMed] [Google Scholar]
- 7.Kuo S, Rhodes RL, Mitchell SL, Mor V, Teno JM. Natural history of feeding-tube use in nursing home residents with advanced dementia. J Am Med Dir Assoc. 2009;10(4):264–70. doi: 10.1016/j.jamda.2008.10.010. Epub 2009 Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teno JM, Mitchell SL, Kuo SK, Gozalo PL, Rhodes RL, Lima JC, Mor V. Decision-making and outcomes of feeding tube insertion: a five-state study. J Am Geriatr Soc. 2011;59(5):881–6. doi: 10.1111/j.1532-5415.2011.03385.x. Epub 2011 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palecek EJ, Teno JM, Casarett DJ, Hanson LC, Rhodes RL, Mitchell SL. Comfort feeding only: a proposal to bring clarity to decision-making regarding difficulty with eating for persons with advanced dementia. J Am Geriatr Soc. 2010;58(3):580–4. doi: 10.1111/j.1532-5415.2010.02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danis M. Stopping nutrition and hydration at the end of life. Waltham, MA: UpToDate; 2013. [Google Scholar]
- 11.Ferris FD, von Gunten CF, Emanuel LL. Competency in end-of-life care: last hours of life. J Palliat Med. 2003;6(4):605–13. doi: 10.1089/109662103768253713. [DOI] [PubMed] [Google Scholar]
- 12.Pasman HR, Onwuteaka-Philipsen BD, Kriegsman DM, Ooms ME, Ribbe MW, van der Wal G. Discomfort in nursing home patients with severe dementia in whom artificial nutrition and hydration is forgone. Arch Intern Med. 2005;165(15):1729–35. doi: 10.1001/archinte.165.15.1729. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SL, Teno JM, Kiely DK, Shaffer ML, Jones RN, Prigerson HG, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–38. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Steen JT, Lane P, Kowall NW, Knol DL, Volicer L. Antibiotics and mortality in patients with lower respiratory infection and advanced dementia. J Am Med Dir Assoc. 2012;13(2):156–61. doi: 10.1016/j.jamda.2010.07.001. Epub 2010 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Steen JT, Albers G, Licht-Strunk E, Muller MT, Ribbe MW. A validated risk score to estimate mortality risk in patients with dementia and pneumonia: barriers to clinical impact. Int Psychogeriatr. 2011;23(1):31–43. doi: 10.1017/S1041610210001079. Epub 2010 Jul 26. [DOI] [PubMed] [Google Scholar]
- 16.Van der Steen JT, Ooms ME, van der Wal G, Ribbe MW. Pneumonia: the demented patient’s best friend? Discomfort after starting or withholding antibiotic treatment. J Am Geriatr Soc. 2002;50(10):1681–8. doi: 10.1046/j.1532-5415.2002.50460.x. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Steen JT, Pasman HR, Ribbe MW, Van Der Wal G, Onwuteaka-Philipsen BD. Discomfort in dementia patients dying from pneumonia and its relief by antibiotics. Scand J Infect Dis. 2009;41(2):143–51. doi: 10.1080/00365540802616726. [DOI] [PubMed] [Google Scholar]
- 18.Givens JL, Jones RN, Shaffer ML, Kiely DK, Mitchell SL. Survival and comfort after treatment of pneumonia in advanced dementia. Arch Intern Med. 2010;170(13):1102–7. doi: 10.1001/archinternmed.2010.181. Erratum in: Arch Intern Med 2011;171(3):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Twycross R, Wilcock A. Palliative care formulary (PCF4) 4th ed. Nottingham, UK: Palliativedrugs.com Ltd; 2011. [Google Scholar]
- 20.Néron A. Arrêt des médicaments en soins palliatifs. In: Association des pharmaciens des établissements de santé du Québec, editor. Guide pratique des soins palliatifs. 4th ed. Montreal, QC: Association des pharmaciens des établissements de santé du Québec;; 2008. pp. 337–53. [Google Scholar]
- 21.Marik PE. Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract. 2010;38(1):35–42. doi: 10.3810/hp.2010.02.276. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs-Lacelle S, Hadjistavropoulos T. Development and preliminary validation of the Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC) Pain Manag Nurs. 2004;5(1):37–49. doi: 10.1016/j.pmn.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Aubin M, Verreault R, Savoie M, LeMay S, Hadjistavropoulos T, Fillion L, et al. Validité et utilité clinique d’une grille d’observation (PACSLAC-F) pour évaluer la douleur chez des aînés atteints de démence vivant en milieu de soins de longue durée. Can J Aging. 2008;27(1):45–55. doi: 10.3138/cja.27.1.045. [DOI] [PubMed] [Google Scholar]
- 24.Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4(1):9–15. doi: 10.1097/01.JAM.0000043422.31640.F7. [DOI] [PubMed] [Google Scholar]
- 25.Parsons C, Hughes CM, Passmore AP, Lapane KL. Withholding, discontinuing and withdrawing medications in dementia patients at the end of life: a neglected problem in the disadvantaged dying? Drugs Aging. 2010;27(6):435–49. doi: 10.2165/11536760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Sykes N, Thorns A. Sedative use in the last week of life and the implications for end-of-life decision making. Arch Intern Med. 2003;163(3):341–4. doi: 10.1001/archinte.163.3.341. [DOI] [PubMed] [Google Scholar]
- 27.Bennett M, Lucas V, Brennan M, Hughes A, O’Donnell V, Wee B. Using anti-muscarinic drugs in the management of death rattle: evidence-based guidelines for palliative care. Palliat Med. 2002;16(5):369–74. doi: 10.1191/0269216302pm584oa. [DOI] [PubMed] [Google Scholar]
- 28.Quinn-Lee L, Gianlupi A, Weggel J, Moch S, Mabin J, Davey S, et al. Use of oxygen at the end of life: on what basis are decisions made? Int J Palliat Nurs. 2012;18(8):369–70. 372. doi: 10.12968/ijpn.2012.18.8.369. [DOI] [PubMed] [Google Scholar]