Figure 3. TRPM2 is required for VEGF-induced c-Src activation and VE-cadherin phosphorylation on Tyr-658 and Tyr-731.
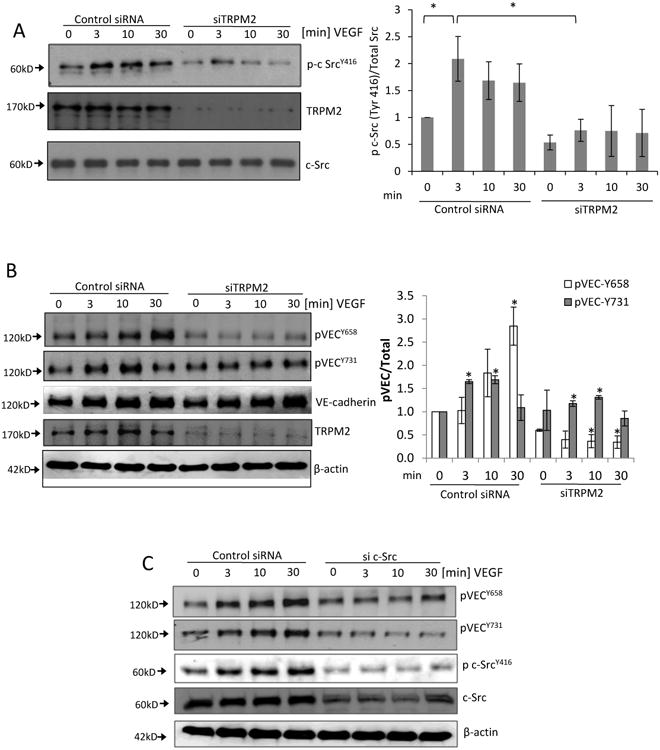
A) Immunoblot showing time-dependent phosphorylation of c-Src at active site Tyr-416 induced by VEGF stimulation of ECs. Maximum phosphorylation was observed at 3 min of VEGF stimulation. Transfection of ECs with TRPM2 siRNA to suppress TRPM2 expression significantly reduced phosphorylation of c-Src at Tyr-416. Mean of n=5 different immunoblots is shown in bar graph (SEM). *p<0.05.
B) Representative Western blots of lysates of ECs transfected with TRPM2 siRNA and then stimulated with VEGF for different times. VEGF induced the phosphorylation of VE-cadherin at Tyr-658 and Tyr-731 peaking at 30 min and 10 min, respectively. Silencing of TRPM2 suppressed VEGF-induced VE-cadherin phosphorylation at both sites. Bar graph shows mean responses of n=3 experiments (SEM) for Tyr-658 and Tyr-731 phosphorylation. *p<0.05 compared to the respective time points in the control group.
C) Immunoblots of human ECs transfected with c-Src siRNA to suppress Src expression, followed by stimulation with VEGF. c-Src knockdown reduced phosphorylation of VE-cadherin on Tyr-658 and Tyr-731. Representative result is shown from two indpependent epxeriments.
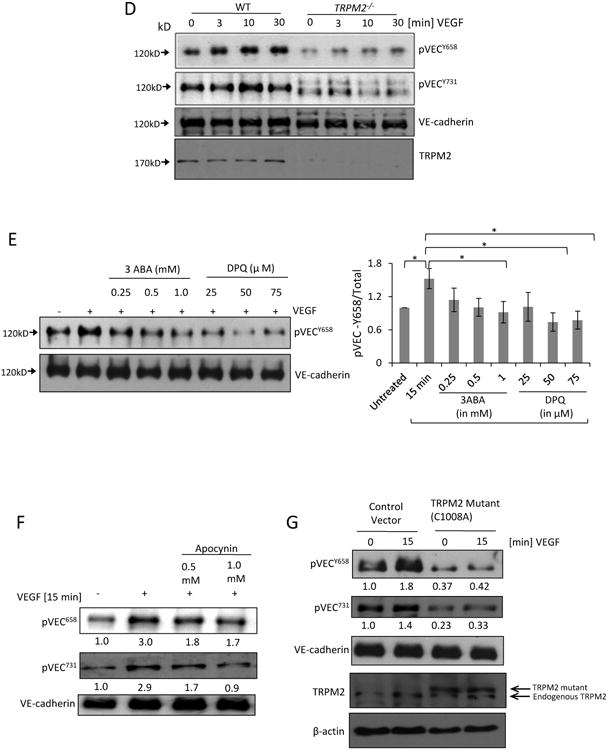
D) Mouse lung vascular ECs obtained from wild type and TRPM2-/- mice were stimulated with VEGF for different times and cell lysate was immunoblotted with anti-phospho VE-cadherin Tyr-658 and Tyr-731 antibodies. Phosphorylation of VE-cadherin was supressed in TRPM2-/ -mouse ECs compared to wild type mouse ECs.
E) Human ECs were treated with 3 aminobenzamide (3ABA) or (3,4-dihydro-5-[4-(1-piperidinyl)butoxyl]-1(2H)-isoquinolinone) (DPQ) at the indicated concentrations, and stimulated with VEGF for 15 min. Both inhibitors suppressed VEGF-induced VE-cadherin phosphorylation with DPQ being more effective. Bar graph shows mean response from 3 different immunoblots (SEM). *p<0.05.
F-G) Tranfection of TRPM2 mutant C1008A or apocynin (Apo) treatment of ECs suppressed VEGF-induced phosphorylation of VE-cadherin at Tyr-658 and Tyr-731. Normalized band densities are shown at the bottom of each blot.
