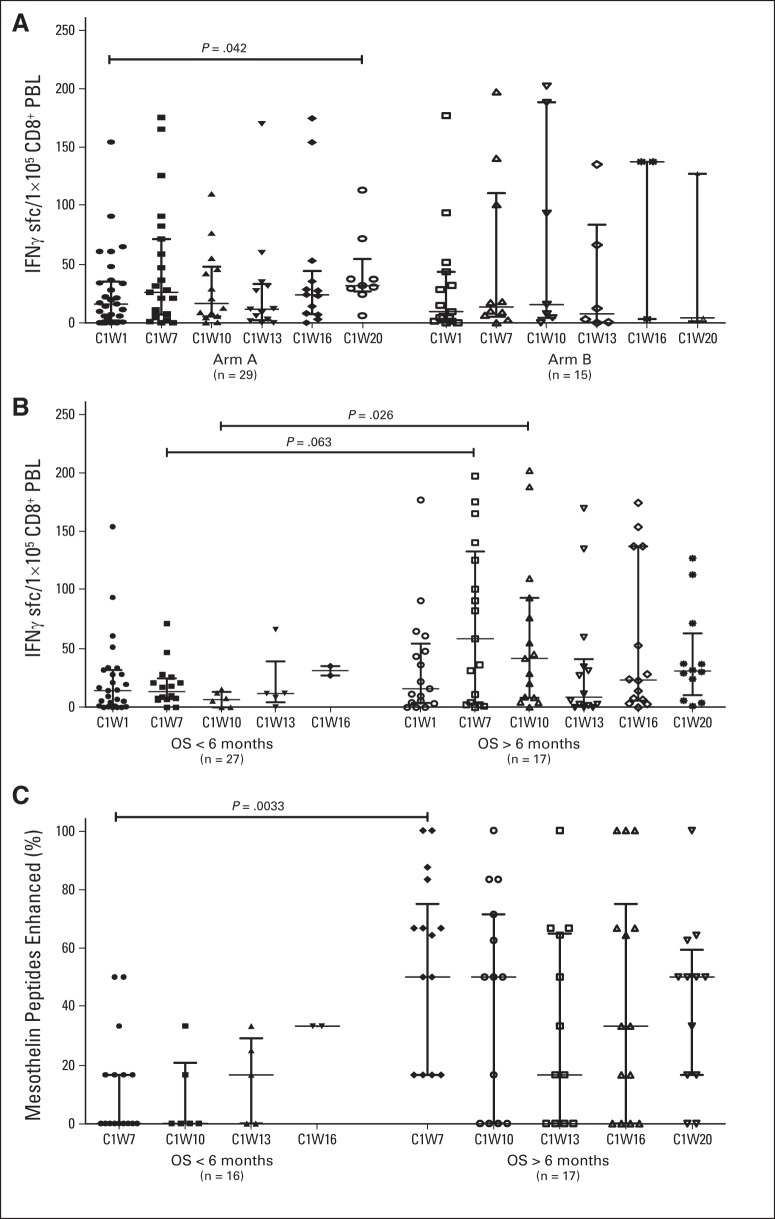
Fig 5.
Mesothelin-specific T-cell responses. Mesothelin-specific CD8 T cells were quantified in peripheral blood lymphocytes (PBLs) using interferon gamma enzyme-linked immunosorbent spot assays at baseline (C1W1), 3 weeks after two treatments with cyclophosphamide (Cy)/GVAX (C1W7), and 3 weeks after each subsequent dose of either CRS-207 (arm A) or Cy/GVAX (arm B; C1W10, C1W13, C1W16, and C1W20) in 44 HLA-A1–, HLA-A2–, and/or HLA-A3–positive patients who received ≥ one treatment of Cy/GVAX. CD8 T-cell responses to mesothelin peptides were considered enhanced when post-treatment T-cell levels were > five per 1 × 105 CD8+ PBLs and increased by ≥ two-fold compared with baseline levels. Cumulative numbers of T cells measured against each HLA-matched mesothelin peptide are reported for each sample. (A) Mesothelin-specific CD8 T-cell levels per 1 × 105 CD8+ PBLs in patients separated by treatment arm. (B) Mesothelin-specific CD8 T-cell levels per 1 × 105 CD8+ PBLs in patients separated by overall survival (OS) > or < 6 months. (C) Size of enhanced post-treatment mesothelin-specific CD8 T-cell repertoire in patients separated by OS > or < 6 months.