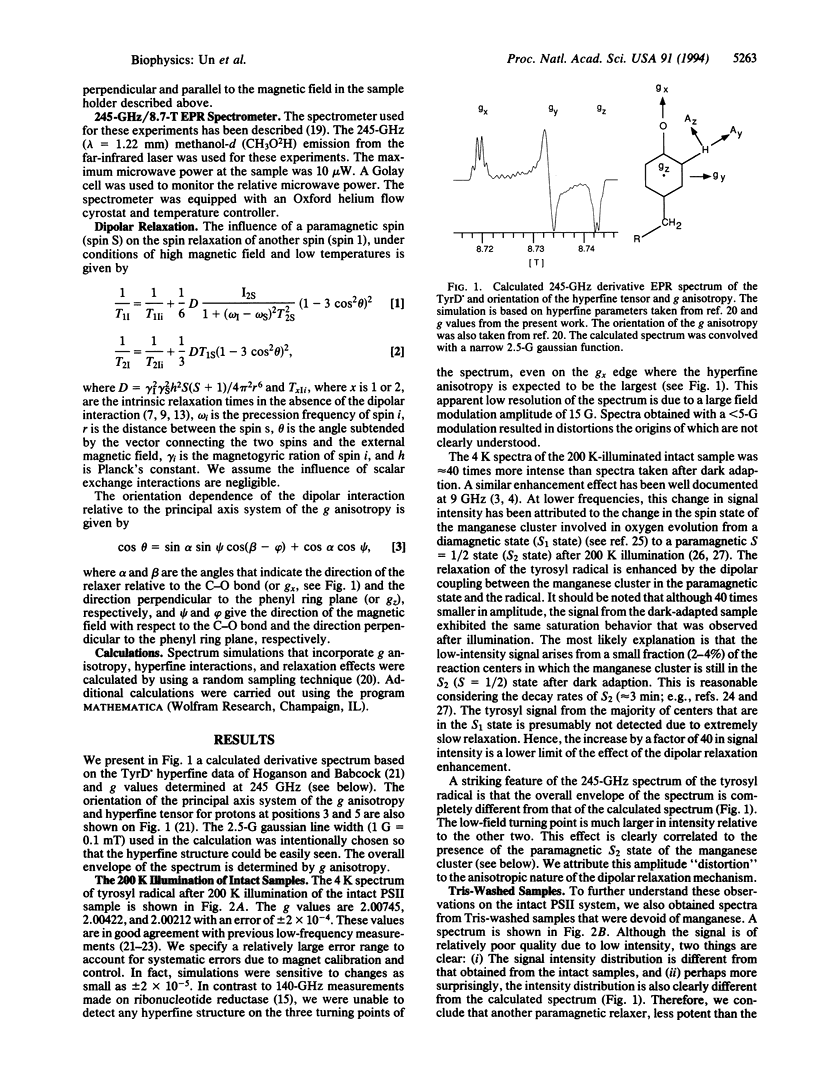
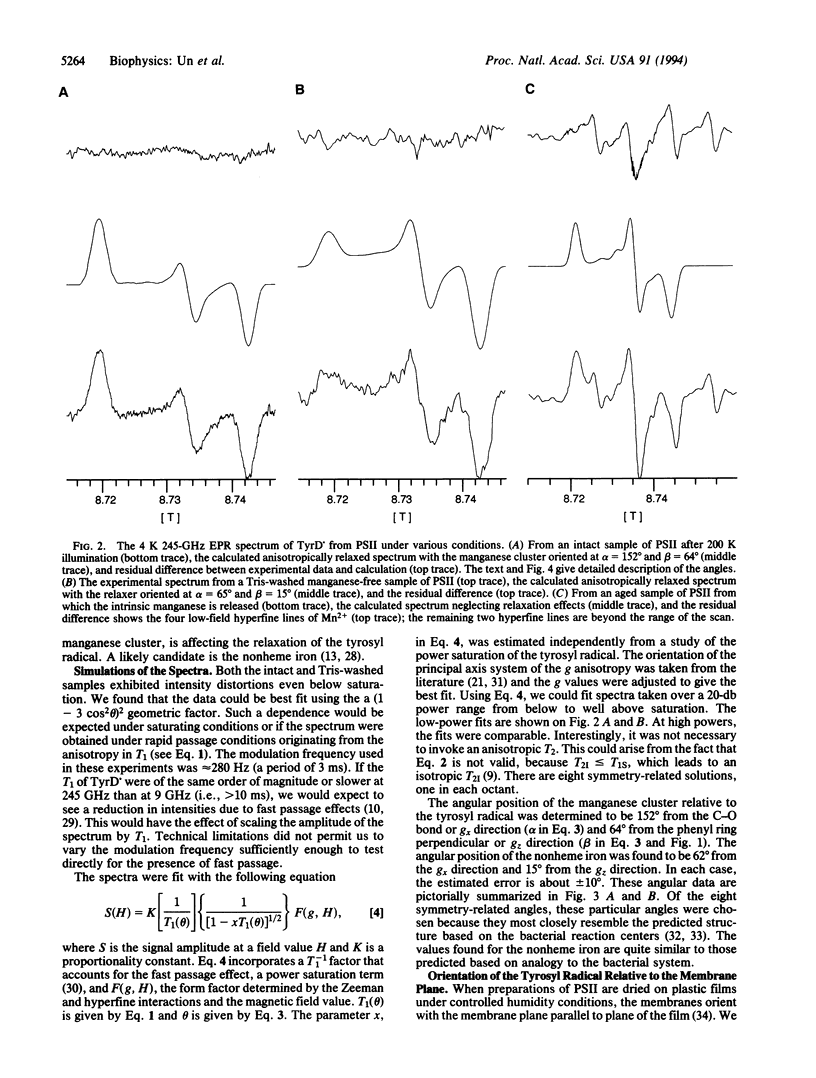
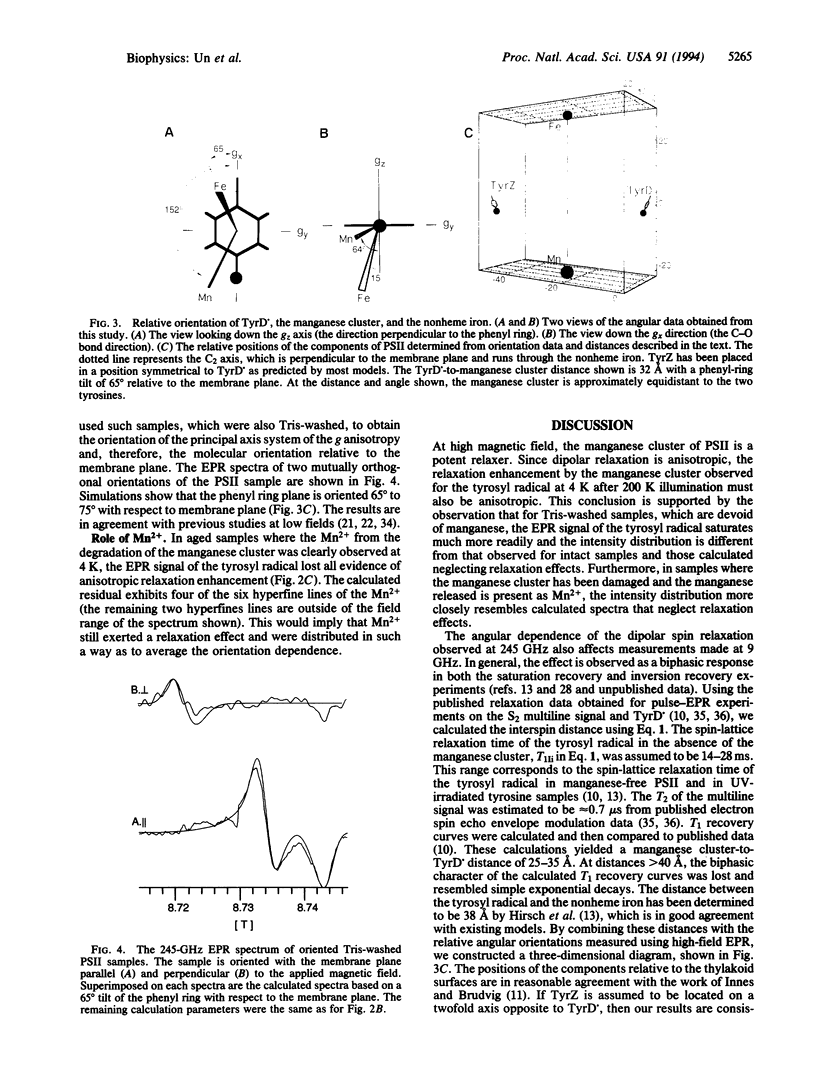
Abstract
The 4 K 245-GHz/8.7-T electron paramagnetic resonance spectrum of the stable tyrosyl radical in photosystem II, known as TyrD., has been measured. Illumination at 200 K enhances the signal intensity of TyrD. by a factor of > 40 compared to the signal obtained from dark-adapted samples. This signal enhancement and the unusual line shape of the TyrD. resonance result from the magnetic dipolar coupling of the radical to the manganese cluster involved in oxygen evolution. The relative angular orientation of the manganese cluster with respect to TyrD. has been determined from line-shape analysis. The resonance arising from TyrD. in Tris-washed manganese-free photosystem II sample is also distorted. This effect probably originates from the influence of the nonheme iron on the spin relaxation of the tyrosyl radical. The relative angular orientation of the nonheme iron has also been determined. Oriented samples were used to determine the angular orientation of TyrD. with respect to the membrane plane. Combining angular data with published distances, we have constructed a three-dimensional picture of the relative positions of TyrD., the manganese cluster, and the nonheme iron. The data suggest a more symmetrical placement of the manganese relative to TyrD. and TyrZ, the tyrosine involved in electron transfer, than is usually assumed in current models of photosystem II.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. doi: 10.1073/pnas.84.16.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock G. T., Barry B. A., Debus R. J., Hoganson C. W., Atamian M., McIntosh L., Sithole I., Yocum C. F. Water oxidation in photosystem II: from radical chemistry to multielectron chemistry. Biochemistry. 1989 Dec 12;28(25):9557–9565. doi: 10.1021/bi00451a001. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Blankenship R. E., Sauer K. Reaction kinetics for positive charge accumulation on the water side of chloroplast photosystem II. FEBS Lett. 1976 Jan 15;61(2):286–289. doi: 10.1016/0014-5793(76)81058-7. [DOI] [PubMed] [Google Scholar]
- Barry B. A. The role of redox-active amino acids in the photosynthetic water-oxidizing complex. Photochem Photobiol. 1993 Jan;57(1):179–188. doi: 10.1111/j.1751-1097.1993.tb02275.x. [DOI] [PubMed] [Google Scholar]
- Britt R. D., Lorigan G. A., Sauer K., Klein M. P., Zimmermann J. L. The g = 2 multiline EPR signal of the S2 state of the photosynthetic oxygen-evolving complex originates from a ground spin state. Biochim Biophys Acta. 1992 Nov 16;1140(1):95–101. doi: 10.1016/0005-2728(92)90024-v. [DOI] [PubMed] [Google Scholar]
- Debus R. J. The manganese and calcium ions of photosynthetic oxygen evolution. Biochim Biophys Acta. 1992 Oct 16;1102(3):269–352. doi: 10.1016/0005-2728(92)90133-m. [DOI] [PubMed] [Google Scholar]
- Dismukes G. C., Siderer Y. Intermediates of a polynuclear manganese center involved in photosynthetic oxidation of water. Proc Natl Acad Sci U S A. 1981 Jan;78(1):274–278. doi: 10.1073/pnas.78.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanella E. L., Gordy W. Electron spin resonance of an irradiated single crystal of L-tyrosine-HC. Proc Natl Acad Sci U S A. 1969 Feb;62(2):299–304. doi: 10.1073/pnas.62.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman G., Leigh J. S., Jr Distance between the visible copper and cytochrome a in bovine heart cytochrome oxidase. Biochemistry. 1985 Apr 23;24(9):2310–2317. doi: 10.1021/bi00330a028. [DOI] [PubMed] [Google Scholar]
- Hirsh D. J., Beck W. F., Innes J. B., Brudvig G. W. Using saturation-recovery EPR to measure distances in proteins: applications to photosystem II. Biochemistry. 1992 Jan 21;31(2):532–541. doi: 10.1021/bi00117a033. [DOI] [PubMed] [Google Scholar]
- Hoganson C. W., Babcock G. T. Protein-tyrosyl radical interactions in photosystem II studied by electron spin resonance and electron nuclear double resonance spectroscopy: comparison with ribonucleotide reductase and in vitro tyrosine. Biochemistry. 1992 Dec 1;31(47):11874–11880. doi: 10.1021/bi00162a028. [DOI] [PubMed] [Google Scholar]
- Innes J. B., Brudvig G. W. Location and magnetic relaxation properties of the stable tyrosine radical in photosystem II. Biochemistry. 1989 Feb 7;28(3):1116–1125. doi: 10.1021/bi00429a028. [DOI] [PubMed] [Google Scholar]
- Moser C. C., Keske J. M., Warncke K., Farid R. S., Dutton P. L. Nature of biological electron transfer. Nature. 1992 Feb 27;355(6363):796–802. doi: 10.1038/355796a0. [DOI] [PubMed] [Google Scholar]
- Nixon P. J., Diner B. A. Aspartate 170 of the photosystem II reaction center polypeptide D1 is involved in the assembly of the oxygen-evolving manganese cluster. Biochemistry. 1992 Jan 28;31(3):942–948. doi: 10.1021/bi00118a041. [DOI] [PubMed] [Google Scholar]
- Prisner T. F., McDermott A. E., Un S., Norris J. R., Thurnauer M. C., Griffin R. G. Measurement of the g-tensor of the P700+. signal from deuterated cyanobacterial photosystem I particles. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9485–9488. doi: 10.1073/pnas.90.20.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B., Vass I., Cedergren E., Styring S. Structure of donor side components in photosystem II predicted by computer modelling. EMBO J. 1990 Jul;9(7):2051–2059. doi: 10.1002/j.1460-2075.1990.tb07372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann J. L., Boussac A., Rutherford A. W. The manganese center of oxygen-evolving and Ca(2+)-depleted photosystem II: a pulsed EPR spectroscopy study. Biochemistry. 1993 May 11;32(18):4831–4841. doi: 10.1021/bi00069a019. [DOI] [PubMed] [Google Scholar]