Abstract
Purpose
Vitiligo prevalence and its associated comorbidities rate have been reported variably among different populations. We aimed to determine the prevalence of vitiligo in Korea along with the baseline rate of comorbidities and compared the risks to the general population using hospital visit information of the total population in Korea.
Materials and Methods
We assessed demographic characteristics of vitiligo patients in Korean population from 2009 to 2011 in a nationwide data from Health Insurance Review Assessment Service. Patients who had at least one visit to Korea's primary, secondary, or tertiary referral hospitals with International Classification of Diseases, 10th Revision, Clinical Modification diagnosis code for vitiligo were identified. As a supplementary study, comorbidities associated with vitiligo were selected for further review to calculate relative risks compared to the general population.
Results
The annual prevalence of vitiligo determined by hospital-visiting rate in Korea was 0.12% to 0.13% over a three year period. In sync with other previous epidemiological studies, there was bimodal distribution among the age groups and no difference between genders. Also, vitiligo in Korean population was associated with various autoimmune/non-autoimmune diseases such as thyroiditis, atopic dermatitis, and psoriasis.
Conclusion
This study was by far the most comprehensive review on prevalence of vitiligo using a data of total population in Korea. The prevalence is within a range of those reported in previous literatures, and increased risk of comorbidities such as thyroid diseases and psoriasis in vitiligo might aid clinicians in the initial work up of vitiligo patients and concurrent follow ups.
Keywords: Vitiligo, epidemiology, prevalence, autoimmune diseases
INTRODUCTION
Vitiligo, an acquired skin disease of progressive depigmentation due to loss of epidermal melanocytes, is reported with varying prevalence over the world. Although there is no wide fluctuation known among various ethnic groups in its prevalence, more evident clinical features of vitiligo in darker-skinned individuals lead to frequent hospital visits and treatment, raising a concern for social stigma.1 In various population-based studies, vitiligo's worldwide prevalence was noted as 0.5% to 1%2 while there were also peaks up to 8%.3 The most recently updated prevalence of vitiligo through screening of more than 50 studies worldwide reported prevalence of vitiligo as between 0.5 and 2%.4 However, as studies using entire population is lacking, preexisting studies have limitations in estimating vitilligo's worldwide prevalence. In South Korea, the total population is about fifty million, and all citizens are obligated to join the National Health Security System. Within this system all information on hospital visits are stored and managed. Therefore, our study using medical information of total population in Korea is based on a good estimated prevalence of certain disease.
Previous studies characterizing vitiligo in Korean population was mostly limited to a survey by tertiary referral hospitals at a single institution.5 A lack of a nation-wide data was a barrier in determining epidemiological data of vitiligo for distribution of patients' age and exact prevalence. Also, it raised a possibility of selection bias through including only severe patients who visited university-level hospitals. Hence to get a broader picture of vitiligo in Korea, we determined prevalence of vitiligo using a nationwide claim database. In addition, we performed a search on prevalence of commonly associated diseases in vitiligo. Autoimmune conditions such as thyroiditis and rheumatoid arthritis are major disease groups that aggravate patients' morbidity and burden of disease.6 Knowledge on prevalence of comorbidities in vitiligo will, therefore, provide insights into future management of the disease.
The purposes of this study were to 1) estimate the prevalence of vitiligo in Korea and 2) compare associated comorbidities in vitiligo with general population of Korea using a nationwide claim database.
MATERIALS AND METHODS
Study population
We assessed demographic characteristics of vitiligo patients in Korean population from 2009 to 2011, selected from a nationwide data in Health Insurance Review Assessment Service (HIRA) of South Korea. The HIRA database is the National Korean Health Insurance claims database. In South Korea, all citizens are obligated to join the National Health Security System which has National Health Insurance Program as one of its three arms.7 The insurance claims database holds all primary to tertiary referral hospitals' data on inpatients and outpatients, including data on diagnosis and treatment costs for claims. It is already actively used as a tool for epidemiological studies in various medical specialties.8,9,10
In our study, patients who had at least one visit to Korea's primary, secondary, or tertiary referral hospitals with a diagnosis of vitiligo according to the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) were identified as vitiligo cases. We then identified comorbidities associated with vitiligo and compared the results with comorbidities present in the general population from 2009 to 2011. Information on the general population was obtained from Korean Statistical Information Service.11
Definition of comorbidity
We collected patients who had at least one claim with principal or secondary diagnosis of following selective diseases that are known to be associated with vitiligo. We classified diseases into two groups: autoimmune and non-autoimmune. First, autoimmune disorders, including lupus erythematosus (ICD-10 codes: L93.0), dermatomyositis (ICD-10 codes: M33.1, M33.9), scleroderma (ICD-10 codes: M34.9, L94.0, L94.1), rheumatoid arthritis (ICD-10 codes: M06), alopecia areata (ICD-10 codes: L63.9), thyroiditis (ICD-10 codes: E06.9), multiple sclerosis (ICD-10 codes: G35), and insulin-dependent diabetes mellitus (ICD-10 codes: E10.61, E10.62) were included. For non-autoimmune disorders, atopic dermatitis (ICD-10 codes: L20), psoriasis (ICD-10 codes: L40), and other comorbidities including sarcoidosis (ICD-10 codes: D86), Tuner syndrome (ICD-10 codes: Q96), anemia (ICD-10 codes: D64.9), vitamin B12 deficiency anemia (ICD-10 codes: D51.9), folate deficiency anemia (ICD-10 codes: D52.9, D52.8) were included.
Statistical analysis
To estimate the prevalence of vitiligo, a total number of physician-diagnosed vitiligo cases were identified in a yearly interval from 2009 to 2011. Afterwards, annual event rates per 100000 patients for each year were obtained by dividing prevalence of one year by total population of the same year. Then, using the raw data from HIRA, we calculated the ratio of event rates for each comorbidities/vitiligo and comorbidities/general population. Calculation of proportion of two different rates was chosen as our statistical analysis method since case number of vitiligo patients stratified into age-groups was not available. Therefore, calculated prevalence of comorbidities in vitiligo patients are not adjusted, but crude rates. While the prevalence rate of vitiligo in general population was determined separately each year, the rates of comorbidities in vitiligo and general population were calculated by summing up the number of cases from 2009 to 2011 and then averaged by three years. After calculating rates, relative risks (RRs) were estimated for comorbidities of vitiligo and general population. RR>1 indicates that comorbidity event was more frequent in vitiligo patients than general population and RR<1 indicates comorbidity event less frequent in vitiligo group than general population. 95% confidence intervals (CIs) for the RRs were also calculated using the following equation: CI of 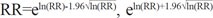 . CI for sex ratio was calculated using exact binomial method.
. CI for sex ratio was calculated using exact binomial method.
RESULTS
Prevalence and demographics
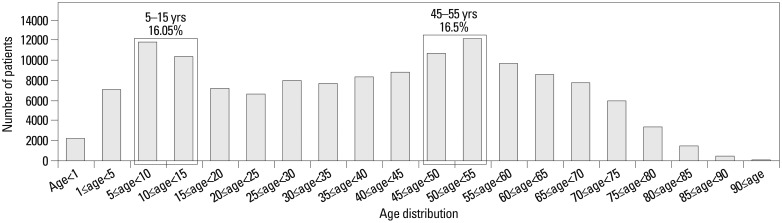
Table 1 shows demographic characteristics of the cases. A total number of physician-diagnosed cases of vitiligo for each year are shown. Prevalence of vitiligo between 2009 and 2011 was approximately 0.13% as a yearly rate with male to female ratio of 1 to 1.31 (95% CI 0.75 to 0.77). Fig. 1 depicts prevalence by 5-year age intervals which shows bimodal distribution of peaks at age 5 to 15 years (16.05%) and 45 to 55 years (16.5%).
Table 1. Demographic of Vitiligo Patients in Korea.
| 2009 | 2010 | 2011 | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Total | 61348 | 0.12 | 63829 | 0.13 | 65224 | 0.13 |
| Gender | ||||||
| Male | 26535 | 0.05 | 27534 | 0.05 | 28289 | 0.05 |
| Female | 34813 | 0.07 | 36295 | 0.07 | 36935 | 0.07 |
| Age | ||||||
| <20 | 16427 | 0.03 | 17046 | 0.03 | 17670 | 0.03 |
| 20-39 | 14277 | 0.03 | 14333 | 0.03 | 14281 | 0.03 |
| 40-59 | 18580 | 0.04 | 19534 | 0.04 | 19799 | 0.04 |
| ≥60 | 12064 | 0.02 | 12916 | 0.03 | 13474 | 0.03 |
Fig. 1. Age distribution of vitiligo patients in Korean population.
Association with comorbidities
Table 2 presents the prevalence of comorbidities in vitiligo patients and it's comparison with general population from year 2009 to 2011. We were not able to match vitiligo cases and general population's control for gender or age. In autoimmune disorders, vitiligo was significantly associated with increased relative risk [RR; (95% CI)] of lupus erythematosus [21.77; (1.02-28.17)], dermatomyositis [1.63; (1.19-3.72)], scleroderma [22.32; (1.02-28.45)], alopecia areata [3.20; (1.00-3.48)], thyroiditis [12.68; (1.00-13.34)], and insulin-dependent diabetes [1.08; (1.01-1.28)]. There were no patients with both vitiligo and rheumatoid arthritis in 2009 to calculate relative risk in comparison with general population and also no vitiligo patients with accompanying multiple sclerosis.
Table 2. Rate per 100000 and RR in Vitiligo Patients Compared to General Population for Associated Comorbidities, Divided into Autoimmune and Non-Autoimmune Disorders.
| Disorders | 2009 | 2010 | 2011 | RR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| Vitiligo | General population | Vitiligo | General population | Vitiligo | General population | |||
| Autoimmune disorders | ||||||||
| Lupus erythematosus | 76.612 | 4.335 | 97.135 | 4.213 | 105.789 | 4.335 | 21.77 | 1.02-28.17 |
| Dermatomyositis | 4.89 | 4.054 | 9.4 | 4.262 | 12.265 | 8.097 | 1.63 | 1.19-3.72 |
| Scleroderma | 99.433 | 4.48 | 109.668 | 4.642 | 107.322 | 5.064 | 22.32 | 1.02-28.45 |
| Rhematoid arthritis | 0 | 506.571 | 1.567 | 446.802 | 1.533 | 439.888 | ||
| Alopecia areata | 570.516 | 270.853 | 1023.046 | 270.338 | 1044.094 | 287.582 | 3.2 | 1-3.48 |
| Thyroiditis | 1478.451 | 186.647 | 2754.234 | 210.319 | 2839.446 | 164.524 | 12.68 | 1-13.34 |
| Multiple sclerosis | 0 | 4.363 | 0 | 0 | 0 | 0 | ||
| Insulin-dependent diabetes | 172.785 | 201.532 | 267.903 | 192.904 | 213.112 | 210.607 | 1.08 | 1.01-1.28 |
| Non-autoimmune disorders | ||||||||
| Atopic dermatitis | 4140.314 | 2091.337 | 6451.613 | 2074.467 | 5947.197 | 1984.981 | 2.7 | 1-2.79 |
| Psoriasis | 1431.18 | 311.457 | 2058.625 | 307.041 | 1833.681 | 308.61 | 5.76 | 1-6.11 |
| Sarcoidosis | 4.89 | 2.565 | 12.533 | 2.736 | 4.6 | 3.159 | 2.61 | 1.24-6.47 |
| Turner syndrome | 11.41 | 2.973 | 20.367 | 2.948 | 16.865 | 3.153 | 5.38 | 1.1-9.92 |
| Anemia | 16.3 | 12.7 | 28.2 | 15.184 | 27.597 | 15.06 | 1.69 | 1.07-2.78 |
| Vitamin B12 deficiency anemia | 11.41 | 5.971 | 15.667 | 6.376 | 15.332 | 6.718 | 2.23 | 1.12-4.29 |
| Folate deficiency anemia | 4.89 | 6.729 | 12.533 | 8.808 | 12.265 | 8.342 | 1.25 | 1.17-2.73 |
RR, relative risk; CI, confidence interval.
Risks of comorbidities in non-autoimmune diseases are as follows: atopic dermatitis [2.70; (1.00-2.79)], psoriasis [5.76; (1.00-6.11)], sarcoidosis [2.61; (1.24-6.47)], Turner syndrome [5.38; (1.10-9.92)], anemia [1.69; (1.07-2.78)], vitamin B12 deficiency anemia [2.23; (1.12-4.29)], and folate deficiency anemia [1.25; (1.17-2.73)].
DISCUSSION
The most comprehensive review of vitiligo prevalence reported thus far by Krüger and Schallreuter4 lists searches of more than 50 studies. While studies collected by Krüger and Schallreuter4 did not follow unified methods and some were based on subgroups rather than general population, it reported a reasonably acceptable prevalence of vitiligo, at around 0.5% to 2%.
Our study is the first study to date that provides a nationally representative data on prevalence of vitiligo in Korea. The prevalence determined by a hospital-visiting rate in Korea was 0.12% to 0.13% over a three year period. The rationale for observing a three year period was to compensate for possible omission of cases who might not visit a clinic in one year period. Since there was no dramatic change in prevalence over the three years, 0.13% seems reliable as a representation of prevalence in Korea. Our reported prevalence is lower than the one reported by Krüger and Schallreuter4 as well as from other western countries. However, because disease burden might vary among different skin-colored population, we deemed it more appropriate to compare our prevalence data with those from other Asian countries.
A study on prevalence of dermatological disorders in Japan, performed as a nationwide, cross-sectional, seasonal, multicenter, hospital-based study, and conducted amongst 64448 cases, reported prevalence of vitiligo as 1.68%.12 But this study was merely a measure of skin disorders in patients attending dermatology clinics, and the actual prevalence might be lower if prevalence was based on general population. Population survey in China, performed as a door-to-door survey in Shaanxi Province for 42833 cases, identified vitiligo prevalence to be 0.093%,13 and another study in the countryside of China's Jiangsu Province reported prevalence between 0.09-0.15%.14 There was also a study with higher prevalence among 47490 people-1.9%-in Anhui province, China.15
Comparing to other Asian countries, Korea's prevalence was lower than that of Japan's, but similar to two of China's epidemiology studies noted thus far. Our study was conducted using a methodology different from China's door-to-door survey, nevertheless, we provided a fresh insight of comparing vitiligo between similar regions, considering genetic factors and skin phototype into account.
For this study, we used data between 2009 and 2011 in the national health claim database obtained from the HIRA of Korea. The HIRA database contains information on entire Korea population that is related to all medical claims in Korea. Furthermore, because a unique identification number is used in the database (Korean Resident Registration Number) assigned at birth, we can rule out omissions or duplications in the records.
Korea is one of few countries with little barriers in entering medical treatment, but it also has a popular medical culture of oriental medicine and alternative medicine (records from these medical communities were not included in our study). Patients attending oriental medical clinics, patients educated with beliefs that vitiligo is incurable and hence refusing to visit hospitals, and non-dermatology trained doctors failing to detect vitiligo, are all contributing factors for lower than expected prevalence of vitiligo which was deduced from medical claims data.
Bimodal distribution was also comparable to other studies reported. The male to female ratio was 1 to 1.31 in Korea, a finding not too far-fetched from equal sex ratio known in literature.16
In the present study, we identified prevalence of comorbidities associated with vitiligo. The largest review, performed in Korea, on comorbidities assessed 1203 vitiligo patients visiting seven university-level tertiary referral hospitals. In the study, there were 16.4% of patients with co-existing thyroiditis, 10.4% with allergic disease (asthma, rhinitis, atopic dermatitis), and 9.7% with diabetes mellitus. However, no extensive searches on other autoimmune diseases were done.17 In a similar skin phenotype group, 133 Japanese patients with generalized vitiligo were investigated for occurrence of autoimmune diseases.18 In these patients, 20.3% had associated autoimmune disorders; Hashimoto's thyroiditis (12%) was the most common disease, followed by alopecia areata (5.3%), and psoriasis (1.5%).
We studied the rate per 100000 of the associated comorbidities, and most of the rates were lower than those shown in previous tertiary level studies. Nevertheless, the general trend of thyroiditis being one of the most prevalent diseases among vitiligo patients was obvious.
In the present study, the rate of atopic dermatitis coinciding with vitiligo was higher than expected, with prevalence of 5.53% in vitiligo patients (data not shown). In a prospective online-questionnaire for vitiligo patients at a single institution showed that out of 2848 patients, a total of 1635 patients (61.8%) had history of at least 1 atopic disease with atopic dermatitis comprising 24.0% of atopic diseases.19 Theoretically, as well as through experience, we believe that the pro-inflammatory status of atopic dermatitis might play as a predisposing factor for vitiligo. Also, constant itching and scratching induced by eczema can induce Koebner effect on vitiligo. However, the prevalence of atopic dermatitis concurrent with vitiligo, based on HIRA data, does raise a concern for overestimated diagnosis. Prescription of topical pimecrolimus or tacrolimus, a frequently used agent for localized vitiligo, is classified as an uninsured drug in Korea to block their usage in off-label conditions, and diagnostic code of atopic dermatitis added to vitiligo patients for insurance claim is not uncommon. Also, with Korea's increasing interest in outward appearances, awareness and screening of atopic dermatitis amongst Korean parents is sometimes beyond a necessary need. Children with hardly visible atopic lesions visit dermatology clinics with concerns of lesions progressing in the future. Because the data used could not exclude these minor cases, overestimation of atopic dermatitis is entirely possible.
Another commonly associated comorbidities is psoriasis. However, association between vitiligo and psoriasis is controversial. In a ten year retrospective study of comorbidities of vitiligo, psoriasis was the most common comorbidity in Asian population and the second most common disease in total population, followed by thyroiditis.1 On the other hand, a review of reports on vitiligo and psoriasis from 1968 to 2010 revealed a relatively similar rate of psoriasis in vitiligo patients compared to the rate of psoriasis in general population, suggesting that coexistence of psoriasis and vitiligo is probably due to coincidence.20 Yet, while the crude prevalence of psoriasis in general population of Korea in our data was 0.3% (data not shown), which is a rate close to the expected prevalence of psoriasis in Korea,21 the prevalence of psoriasis in vitiligo patients was higher at 1.78% (data not shown).
Interestingly, adjusted RR of vitiligo in psoriatic patients using national database in Taiwan was 5.94,22 and our RR of psoriasis in vitiligo patients was 5.76. Although direct comparison of two RRs are not possible due to different methodologies used in each studies, increased rate of both vitiligo and psoriasis when associated with each other suggests common genetic locus in major histocompatibility complex (MHC) of both diseases23 and shared pathogenic pathways through Th1, and Th17 pathways.24,25
In addition, the majority of autoimmune diseases showed higher RRs than non-autoimmune disease group. Phenomenon observed in our study could be due to involvement of NALP1 in increasing susceptibility of autoimmune or autoinflammatory diseases in vitiligo, but more studies are needed for confirmation.26
Our nationwide data did not perform stratification of patients according to gender, age, and subtypes of vitiligo (generalized, segmental). Usually, generalized vitiligo is associated with higher prevalence of autoimmune diseases, since generalized vitiligo shares genetic susceptibility loci with other autoimmune diseases.27 Although there is a growing consensus that there might be an overlapping mechanism between segmental and non-segmental vitiligo, future population-based study on difference in associated comorbidities for varying types of vitiligo is needed.
There are limitations of this study which should be taken into consideration. First, the prevalence of vitiligo and comorbidities in vitiligo and general populations was solely based on coding in HIRA database which raises a concern for a reporting bias. A possibility of under-reporting due to failure of patient visiting a clinic and lack of awareness exists. However, since barrier to seeking medical treatment is not strong in Korea, we do not believe that such factors had significant impact on overall prevalence of our study. Furthermore, since it was not possible to differentiate diagnostic codes entered by dermatologists and non-dermatologists, inaccurate coding for vitiligo and clinicians failure to detect comorbidities beyond their scope of specialty might have resulted in some under-reporting.
Moreover, most of vitiligo patients in Korea undergo treatment of narrowband UVB or 308-nm excimer laser at a tertiary referral hospital, making it more frequent regular check-ups and hospital visits. We believe that such referrals might artificially increase comorbidity rate.
Lastly, statistical method applied in our work provides only a crude rate of associated comorbidities' prevalence and relative risk, since we could not stratify the patients into age groups. Inability to extract age-specified groups due to limited data available through HIRA probably accounts for 95% CI with not so narrow range. In spite of these limitations, however, our study deserves attention as it was the first nationwide data-based epidemiologic study to determine the prevalence of vitiligo in Korea, showing a crude nevertheless generalized trend of comorbidities associated with vitiligo.
In conclusion, we studied a nationwide prevalence of vitiligo in Korea with population-based data over a three year period. The total study population covered approximately 50593516 annual cases for 3 consecutive years, and it is by far the most comprehensive review on prevalence of vitiligo. Our noted prevalence is within the range of those reported in previous literatures, and there was also bimodal distribution with no difference in gender. Also, we found that prevalence of vitiligo in South Korea was associated with significantly increased risk of comorbidities such as thyroid diseases and psoriasis. The finding of these associations should aid clinicians in initial work up of vitiligo patients and concurrent follow ups.
ACKNOWLEDGEMENTS
This study has been funded by a grant sponsored by MSD in year 2012.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Sheth VM, Guo Y, Qureshi AA. Comorbidities associated with vitiligo: a ten-year retrospective study. Dermatology. 2013;227:311–315. doi: 10.1159/000354607. [DOI] [PubMed] [Google Scholar]
- 2.Taïeb A, Picardo M; The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27–35. doi: 10.1111/j.1600-0749.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 3.Shah H, Mehta A, Astik B. Clinical and sociodemographic study of vitiligo. Indian J Dermatol Venereol Leprol. 2008;74:701. doi: 10.4103/0378-6323.45144. [DOI] [PubMed] [Google Scholar]
- 4.Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206–1212. doi: 10.1111/j.1365-4632.2011.05377.x. [DOI] [PubMed] [Google Scholar]
- 5.Park SY, Youn JI, Lim SD. A clinical study of 217 cases of vitiligo. Korean J Dermatol. 1981;19:145–152. [Google Scholar]
- 6.Taïeb A, Picardo M. Epidemiology, definitions and classification. In: Picardo M, Taïeb A, editors. Vitiligo. Berlin: Springer; 2010. pp. 13–24. [Google Scholar]
- 7.Song YJ. The South Korean Health Care System. JMAJ. 2009;52:206–209. [Google Scholar]
- 8.Lee YK, Ha YC, Park C, Yoo JJ, Shin CS, Koo KH. Bisphosphonate use and increased incidence of subtrochanteric fracture in South Korea: results from the National Claim Registry. Osteoporos Int. 2013;24:707–711. doi: 10.1007/s00198-012-2016-8. [DOI] [PubMed] [Google Scholar]
- 9.Park SJ, Choi NK, Park KH, Woo SJ. Five year nationwide incidence of rhegmatogenous retinal detachment requiring surgery in Korea. PLoS One. 2013;8:e80174. doi: 10.1371/journal.pone.0080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YK, Jang S, Jang S, Lee HJ, Park C, Ha YC, et al. Mortality after vertebral fracture in Korea: analysis of the National Claim Registry. Osteoporos Int. 2012;23:1859–1865. doi: 10.1007/s00198-011-1833-5. [DOI] [PubMed] [Google Scholar]
- 11.Korean Statistical Information Service. Summary of Census Population. [accessed on 2013 December 23]. Available at: http://www.kosis.kr.
- 12.Furue M, Yamazaki S, Jimbow K, Tsuchida T, Amagai M, Tanaka T, et al. Prevalence of dermatological disorders in Japan: a nationwide, cross-sectional, seasonal, multicenter, hospital-based study. J Dermatol. 2011;38:310–320. doi: 10.1111/j.1346-8138.2011.01209.x. [DOI] [PubMed] [Google Scholar]
- 13.Lu T, Gao T, Wang A, Jin Y, Li Q, Li C. Vitiligo prevalence study in Shaanxi Province, China. Int J Dermatol. 2007;46:47–51. doi: 10.1111/j.1365-4632.2006.02848.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B. Clinical Dermatology. 3rd ed. Nanjing: Jiangsu Scientific Press; 2001. [Google Scholar]
- 15.Xu YY, Ye DQ, Tong ZC, Hao JH, Jin J, Shen SF, et al. An epidemiological survey for four skin diseases in Anhui. Chin J Dermatol. 2002;35:406–407. [Google Scholar]
- 16.Hann SK, Nordlund JJ. A Monograph on the Basic and Clinical Science. Oxford: Blackwell Science Ltd.; 2000. [Google Scholar]
- 17.Yu HJ, Park KC, Ahn JS, Lim JG, Kwon TE, Koh WS, et al. Clinical study of vitiligo. Korean J Dermatol. 1998;36:1037–1042. [Google Scholar]
- 18.Narita T, Oiso N, Fukai K, Kabashima K, Kawada A, Suzuki T. Generalized vitiligo and associated autoimmune diseases in Japanese patients and their families. Allergol Int. 2011;60:505–508. doi: 10.2332/allergolint.11-OA-0303. [DOI] [PubMed] [Google Scholar]
- 19.Silverberg JI, Silverberg NB. Association between vitiligo and atopic disorders: a pilot study. JAMA Dermatol. 2013;149:983–986. doi: 10.1001/jamadermatol.2013.4228. [DOI] [PubMed] [Google Scholar]
- 20.Sawchuk M, Spano F, Loo WJ, Guenther L. The coexistence of psoriasis and vitiligo: a review. J Cutan Med Surg. 2012;16:300–305. doi: 10.1177/120347541201600505. [DOI] [PubMed] [Google Scholar]
- 21.Youn JI. Psoriasis in Korean. Korean J Dermatol. 2012;50:387–402. [Google Scholar]
- 22.Tsai TF, Wang TS, Hung ST, Tsai PI, Schenkel B, Zhang M, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011;63:40–46. doi: 10.1016/j.jdermsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhu KJ, Lv YM, Yin XY, Wang ZX, Sun LD, He SM, et al. Psoriasis regression analysis of MHC loci identifies shared genetic variants with vitiligo. PLoS One. 2011;6:e23089. doi: 10.1371/journal.pone.0023089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol. 2011;36:292–297. doi: 10.1111/j.1365-2230.2010.03972.x. [DOI] [PubMed] [Google Scholar]
- 25.Basak PY, Adiloglu AK, Ceyhan AM, Tas T, Akkaya VB. The role of helper and regulatory T cells in the pathogenesis of vitiligo. J Am Acad Dermatol. 2009;60:256–260. doi: 10.1016/j.jaad.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 26.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 27.Spritz RA. Shared genetic relationships underlying generalized vitiligo and autoimmune thyroid disease. Thyroid. 2010;20:745–754. doi: 10.1089/thy.2010.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]