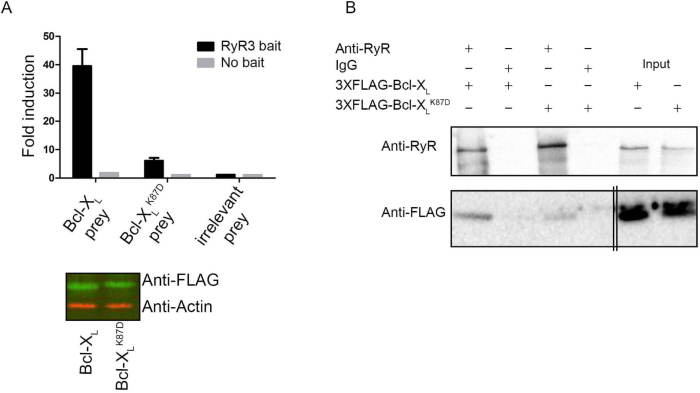
Figure 3. The Bcl-XLK87D mutant is impaired in RyR3 binding.
(A) Top: Representative example of a MAPPIT experiment. The binding is shown as fold induction value, calculated by dividing the average luciferase value of erythropoietin-stimulated cells by the average of non-stimulated cells. Binding of Bcl-XL (Bcl-XL prey), the Bcl-XLK87D mutant (Bcl-XLK87D prey) or irrelevant prey control (SV40 large T antigen) to the RyR3 domain (RyR3 bait) and as negative control the bait vector without RyR3 (No bait) are shown. Fold induction values at least 4 times higher than the irrelevant prey control are considered as bona fide protein-protein interactions. Values represent the average of three repeats within the same experiment ± S.D. All experiments were independently performed at least three times. Bottom: Odyssey Western blot analyses staining for the FLAG tag of the prey vector containing Bcl-XL or the Bcl-XLK87D mutant fusion proteins (green) or for actin (red) as a loading control. All samples were run using the same experimental conditions on the same gel/blot. The uncropped image is shown in Supplementary Fig. 1B. (B) Co-immunoprecipitations were performed in HEK RyR3 cells transiently overexpressing 3XFLAG-Bcl-XL or 3XFLAG-Bcl-XLK87D similarly as in Fig. 1A. Non-specific IgG antibodies were applied as negative controls. These experiments were performed at least three times utilizing each time independently transfected and freshly prepared HEK RyR3 cell lysates. All samples were run using the same experimental conditions and were derived from the same gel/blot, i.e. 3-8% tris-acetate gels for RyRs and 4-12% bis-tris gels for 3xFLAG-Bcl-XL. The double lines indicate that an additional empty lane separating the immunoprecipitated samples and the input samples was removed for the 3XFLAG-Bcl-XL blot. The uncropped image is shown in Supplementary Fig. 1C.