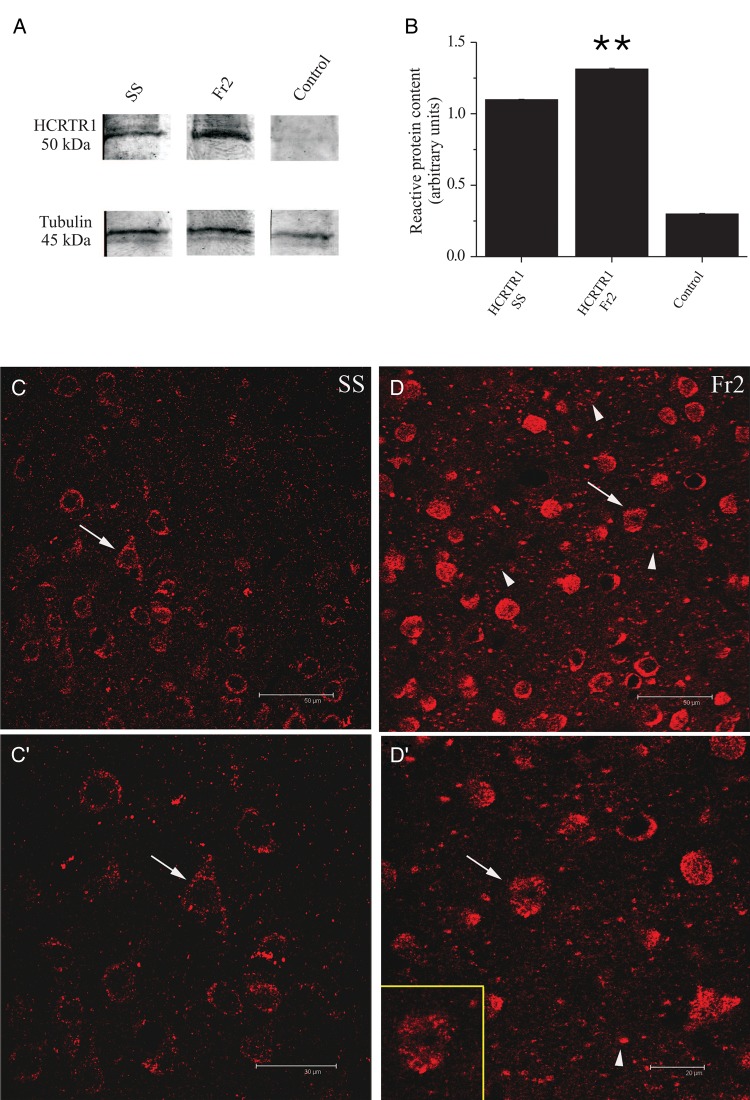
Figure 8.
Western blot and immunocytochemical analysis of HCRTR-1 expression. (A) HCRTR-1 expression in membrane fractions of SS (lane 1) and Fr2 (lane 2). Data were obtained by using Mem-PER. The antibody against HCRTR-1 recognized a specific band with an apparent molecular mass of 50 kDa, consistent with the expression of the native HCRTR-1 (according to NP_001516.1 and Karteris et al. 2005). Equal protein loading was assayed by evaluating the total α-tubulin with a monoclonal anti-α-tubulin antibody as a loading control (lower panels; see Materials and Methods). Controls carried out using preadsorbed HCRTR-1 antiserum and with omission of primary HCRTR-1 antibody were completely negative (lane 3). (B) Comparison of the average intensity of the western blot bands calculated for 3 representative experiments, in the indicated conditions. Optical intensity was detected and analyzed as detailed in Materials and Methods. The immunoreactivity obtained with anti-HCRTR-1 was normalized to the one obtained with anti-α-tubulin, and the resulting ratios were plotted for both SS and Fr2, as indicated. In these tests, HCRTR-1 gave a mean value of 1.31 ± 0.006 for Fr2 and 1.10 ± 0.004 for SS (**P < 0.01; comparison between HCRTR-1 in SS and HCRTR-1 in Fr2). (C), (C′), (D), and (D′) illustrate the immunocytochemical analysis of HCRTR-1 expression. In layer V of SS (C), the HCRTR-1 granular immunoreactivity was mainly detected at the level of neuronal cell bodies sometimes clearly identifiable as pyramidal neurons, based on morphology (arrows). (C′) gives a higher magnification detail of (C). In layer V of Fr2 (D), the HCRTR-1 positive (+) signal was considerably more intense than in SS and was detectable not only in numerous cell bodies (arrows and inset in D′), but also in neuropilar processes of different caliber (arrowheads). (D′) gives a higher magnification detail of (C). Scale bars: 50 μm (C, D); 30 μm (C′); 20 μm (D′).